Key points:
- Uptake of the HPV vaccines in Scotland exceeds 90% for all doses;
- The vaccine is associated with significant reduction in five high-risk HPV types, which contribute to 90% of cervical cancers in Scotland;
- High uptake of vaccine associated with reductions in HPV 16 and HPV in unvaccinated women owing to herd immunity effect;
- Vaccination is associated with significant reductions in both low and high-grade cervical intraepithelial neoplasia;
- Cervical screening programmes must change to accommodate increasingly vaccinated women where disease is rare;
- Quadrivalent vaccine is associated with significant reduction of genital wart prescriptions in young women in Scotland.
Introduction
Immunisation against the two human papillomavirus (HPV) genotypes, HPV 16 and 18, found in 70% of cervical cancers, promises a substantial reduction in high-grade cervical intraepithelial neoplasia (CIN) by 67% and cervical cancers by 70%[1]
. In the UK, HPV vaccination of girls aged 12–13 years as part of the school immunisation programme started in 2008, together with a three-year ‘catch-up’ programme’ for girls aged up to 18 years[2]
. This was introduced to expand the immunised cohort and reduce the lag time to achieving the benefits of vaccination. Uptake rates for three doses in Scotland are high; almost 90% of girls eligible in the school programme and 65.5% in the catch-up cohort received all three doses with equitable uptake by deprivation score[2]
. Although the bivalent vaccine (Cervarix; GlaxoSmithKline) was the vaccine initially offered to schoolgirls, from September 2012, the Joint Committee for Vaccination and Immunisation recommended quadrivalent vaccine (Gardasil; Merck) because it was shown to be more cost effective owing to its additional benefit in protecting against genital warts.
Estimating the pre-immunisation prevalence of circulating HPV and distribution of HPV types is crucial in understanding the subsequent impact of HPV vaccination. Utilising a range of bio-specimens, the prevalence of any HPV in young women aged 20–21 years in Scotland was 32.2% for urine, 39.5% for a self-taken swab and 49.4% for liquid-based cytology specimens, the latter being obtained through cervical screening[3]
. Infection with vaccine-specific types (HPV 16 and 18) or those associated with cross-protection (HPV 31, 33, 45) was common. Understanding of the pre-immunisation prevalence of HPV in young women has now enabled Scotland to assess the early effect of vaccination as the first highly vaccinated cohorts of individuals enter the screening programme.
The advent of immunisation against the two most common high-risk types of HPV (HPV 16 and 18) is beginning to profoundly alter the prevalence of HPV 16 and 18, as well as HPV 31, 33 and 45, in the Scottish population[4],[5]
. Consequently, the severity of CIN, the precursor of invasive cervical cancer, is also changing in young women attending for cervical screening, with a significant reduction in both low and high-grade abnormalities[4]
.
As Scotland begins cervical screening at the age of 20 years (although this was amended to the age of 25 years in June 2016 to ensure consistency with other UK countries)[6]
, girls immunised as part of the catch-up cohort have been entering the cervical screening programme since 2011. As a result, Scotland is almost uniquely placed to determine the impact of immunisation on cervical HPV infection, incidence of CIN and genital warts in young women using national datasets, which can be linked effectively.
Sources and selection criteria
PubMed (National Library of Medicine) was the main electronic database that was searched, using the search term ‘HPV’, in combination with ‘vaccine’ and/or ‘impact’. Similar separate searches were made with ‘cervical intraepithelial neoplasia’ in combination with ‘prevention’ and/or ‘screening’ to ensure that no articles were missed. Searches were limited to links to ‘full-text only’, ‘humans’, ‘English-language articles’, ‘males and females’, and ‘all adult ages’. The ‘Related Articles’ function of PubMed was used to cross-check for additional relevant studies. There was no restriction on the age of the literature, but the studies generated were primarily from the 1990s to 2017. Titles and abstracts identified in the broad search were examined, and the studies included in the review if they were directly relevant to HPV vaccine and one of the ‘trigger’ areas. Retrieved articles were also reviewed for relevant citations. Research studies published only in abstract form were excluded. Finally, each article was reviewed for quality and clinical relevance.
The vaccines and their safety profile
All licensed HPV vaccines include L1-stage virus-like particles (VLPs) as antigens, which have been shown to be particularly immunogenic and stimulate neutralising antibodies to the vaccine-specific HPV types[7]
. The quadrivalent vaccine employs a traditional alum-based adjuvant, with high levels of neutralising antibody generated against HPV 6, 11, 16 and 18. The bivalent HPV vaccine comprises a novel AS04 adjuvant, which has been shown to stimulate NF-κB with increased cytokine production as a result of increased numbers of activated, antigen-loaded dendritic cells and monocytes, further increasing the activation of antigen-specific T cells[8]
. Animal models suggest that only very low levels of antibody are required to be protective, and that immunity is expected to last for 20–30 years[9]
.
The safety profile of both vaccines is excellent, with the most commonly reported side effect being pain at the injection site[10]
. A case series of postural-orthostatic tachycardia syndrome (POTS) in 25 women in Denmark suggested a temporal association with quadrivalent HPV vaccine[11]
. However, a case-control study identified that women with suspected severe adverse events were more likely to exhibit healthcare-seeking behaviour prior to HPV immunisation[12]
. Furthermore, in 2015/2016, the European Medicines Agency, which evaluates medicinal products for use in Europe, conducted its own review of the evidence and stated that the HPV vaccines do not cause POTS[13]
.
Implementation of the vaccine programme
Since 2008, school-based uptake of both the bivalent and quadrivalent HPV vaccines in girls aged 12–13 in Scotland has been impressive, with vaccine uptake sustained at levels exceeding 90%[2]
. Furthermore, a three-year (from September 2008 to 2011) catch-up campaign offered vaccine to all girls aged 13–17, with uptake recorded between 80% and 30% in younger and older girls at age of vaccination, respectively[14]
. In order to estimate vaccine impact, it is important to ascertain the effect of the vaccination programme on the whole population, with particular focus on the age group where these changes will be initially observed. In Scotland, cervical screening was offered three-yearly to all women aged 20–60 years, until June 2016. Therefore, it is one of the few countries in the world able to detect an early impact of the vaccine through population-based surveillance[4],[5]
.
While the girls’ programme confers indirect protection to heterosexual males, men who have sex with men (MSM) receive little benefit from it. At the behest of the Joint Committee for Vaccination and Immunisation, Public Health England conducted modelling studies to ascertain whether offering the HPV vaccine to MSM was cost effective. The modelling studies assessed the cost–effectiveness of a targeted HPV vaccination of MSM in genito-urinary medicine and HIV clinics, looking at the impact of vaccination against penile, anal and oropharyngeal (head and neck) cancers, and genital warts[15]
. Evidence suggests that 80–85% of anal cancers and 50% of penile cancers are linked to HPV infection[16]
. Steinau et al. suggest that in their analysis of United States cancer registries, 62% of oropharyngeal squamous cell carcinomas were positive for both HPV 16 and 18[17]
. While prevalence varied significantly by demography, the HPV vaccine should afford a significant protective effect against HPV-driven oropharyngeal cancers, provided that the duration of serologic immunity is maintained for many years[18]
.
In 2017, a selective HPV immunisation programme will be nationally co-ordinated through sexual health and HIV clinics for MSM in Scotland[19]
. This differs from England, where a number of pilot clinics have been offering the vaccine to MSM since June 2016[20]
. The results of the pilot will inform the potential roll out of a national programme that will benefit the whole MSM population in England.
Impact of the HPV vaccines
Both the prophylactic bivalent and quadrivalent vaccines prevent infection with HPV types 16 and 18, and have been shown to induce avid and sustained neutralising antibody responses, while conferring protection against consequent viral-induced CIN[21]
,[22]
. The bivalent HPV vaccine also affords immunological cross-protection against other high-risk oncogenic HPV types that are phylogenetically related to HPV 16 and 18, including HPV 31, 33 and 45 (see Figure 1)[23]
. Cross-protection for quadrivalent vaccine has also been demonstrated against HPV 31 and 33[24]
. Furthermore, among unvaccinated women, HPV 16 and 18 infections were significantly lower in 2013 (21.2%) than in 2009 (30%), suggesting a community, also known as a herd immunity, effect.
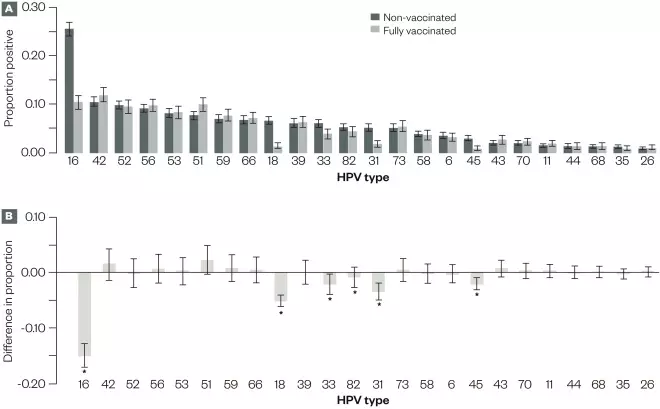
Figure 1: Analyses for 5,715 liquid-based cytology cervical samples from vaccinated and non-vaccinated women, for which valid human papillomavirus (HPV) testing results were available, Scotland, 2009–2013
Source: Data reproduced with permission from Health Protection Scotland
A) Proportion and 95% CIs for samples with positive results for each HPV type.
B) Difference in the proportion positive and associated 95% CIs for the difference between vaccinated and non-vaccinated women, by HPV type. Other than HPV types 16 and 18, the 95% CIs of the difference were corrected for multiple testing using the Bonferroni correction.
*Significant change.
Emerging data from population-based surveillance of HPV vaccination programmes suggest that vaccination is strongly associated with a reduction in both low- and high-grade cervical abnormalities (CIN of grade 1 or worse) in young women[4],[25]
. In Scotland, there was a 50% reduction in CIN2 and a 55% reduction in CIN3 associated with three doses of the bivalent HPV vaccine. Given that such an impact was demonstrated in women who were vaccinated as part of the catch-up programme, the effect of vaccine on routinely-immunised girls is likely to be profound.
The quadrivalent vaccine, which additionally includes HPV types 6 and 11 (both low risk) that are associated with 85–95% of genital warts, has also been shown to be strongly associated with an 85% and 71% reduction in genital warts in both females and heterosexual males, respectively, in Australia[26]
. Furthermore, in Australia, the 71% decrease in genital warts in heterosexual men was observed prior to the implementation of vaccination of boys in 2013 and is likely due to herd immunity. Modelling data from Australia predict that genital warts will approach elimination in both female and male populations in the coming decades, provided vaccine uptake is maintained at the current level[27]
.
While the reduction of disease is to be welcomed, this will have an adverse effect on the current cervical screening strategy within the UK. As disease prevalence reduces in young women over time, so the positive predictive value (PPV) in detecting disease and key performance indicators for the national colposcopy programme have been affected[28]
. Significant reductions in PPV (16%) and abnormal predictive value (63%) for CIN2+ and the mean colposcopy score (18%) were observed. A significant increase (38%) in the number of women who had to be referred to colposcopy to detect one case of CIN2+ was also shown. These findings have implications for screening, colposcopy referral criteria, colposcopy practice and histology reporting.
A recent study has already shown that there has been a reduction in clinical activity related to abnormal screening referral[29]
. This includes a significant reduction in the proportion of women referred with high-grade dyskaryosis and any grade of dyskaryosis (women referred in 2008/09; 41.2%, 2013/14; 30.7%; linear trend P =<0.01). Furthermore, the number of women with high-grade dyskaryosis more than halved from 533 in 2008/2009 to 233 in 2013/2014. Referral criteria and service provision of colposcopy need to be planned carefully, taking account of the increasing number of HPV-immunised women who will be entering cervical screening programmes worldwide.
Implications for pharmacy prescribing
One of the major strengths of Scottish health data is the ability to perform robust data linkage in a national population, using the Community Health Index (CHI). CHI is a unique identifier that can be used to link the immunisation record with a number of other registers, including cervical screening, hospital admission and community pharmacy prescribing. Information on the use of prescription drugs in primary care is held in the Prescribing Information System database held by the information services division of the Scottish NHS. In Scotland, a data linkage approach has been utilised to link HPV vaccination status with primary care prescription data to assess the effect of both the bivalent vaccine and quadrivalent vaccines on genital wart prescription data as a proxy for genital wart diagnoses (unpublished data). A person was identified as having a genital warts episode if they were prescribed any brand of either podophyllotoxin or imiquimod, which are commonly used to treat genital warts. High uptake of the bivalent vaccine had no effect on genital wart prescriptions in Scotland (relative risk [RR]=0.93; 95% confidence interval [CI]: 0.67–1.29; P =0.658). However, there was preliminary evidence of an effect of the quadrivalent vaccine on genital wart prescriptions in young Scottish women (RR=0.33; 95% CI: 0.14–0.83; P =0.018) and the utilisation of prescriptions data in subsequent years will allow the full effect of the vaccine to be assessed.
Finally, a recently published case report demonstrates some efficacy of the quadrivalent HPV vaccine on recalcitrant cutaneous warts[30]
. Most cutaneous warts are caused by infection with HPV 2, therefore, the case-report author postulates that the clearance of this virus may be because of a potential cross-protection effect from the HPV 6 component of the vaccine. If such observations are demonstrated on a much larger scale, community prescribing of topical salicylic acid and imiquimod for cutaneous warts will decrease significantly in those individuals who have been vaccinated. Also, a herd effect may be observed as causative viruses are eliminated from the younger population.
Conclusion
In the UK, high uptake of both HPV vaccines is associated with a significant reduction in both low and high-risk HPV types in young women. Reductions in cervical disease and genital warts are now being demonstrated, with a decrease in cervical cancers expected within the next few years. Referral criteria and service provision of colposcopy need to be planned carefully to take into account the increasing number of HPV-immunised women who will be entering cervical screening programmes worldwide. The impact of the HPV vaccines on other HPV-driven cancers will not be realised for many more years owing to longer latency of infection and oncogenesis in non-cervical tissues[31]
. However, HPV vaccines offer enormous potential for primary cancer prevention.
Author disclosure and conflict of interest
Kevin G Pollock received funding from Merck for speaking at the International Papillomavirus Conference, Lisbon 2015. No writing assistance was utilised in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Smith JS, Lindsay L, Hoots B et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007;121:621–632. doi: 10.1002/ijc.22527
[2] Sinka K, Kavanagh K, Gordon R et al. Achieving high and equitable coverage of adolescent HPV vaccine in Scotland. J Epidemiol Community Health 2014;68:57–63. doi: 10.1136/jech-2013-202620
[3] Kavanagh K, Sinka K, Cuschieri K et al. Estimation of HPV prevalence in young women in Scotland; monitoring of future vaccine impact. BMC Infect Dis 2013;13:519. doi: 10.1186/1471-2334-13-519
[4] Pollock KG, Kavanagh K, Potts A et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer 2014;111:1824–1830. doi: 10.1038/bjc.2014.479
[5] Kavanagh K, Pollock KG, Potts A et al. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer 2014;110:2804–2811. doi: 10.1038/bjc.2014.198
[6] Health Scotland. (2016) Cervical screening. Available at: http://www.healthscotland.com/topics/health-topics/screening/cervical.aspx (accessed March 2017)
[7] Pedersen C, Petaja T, Strauss G et al. HPV Vaccine Adolescent Study Investigators Network. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health 2007;40:564–571. doi: 10.1016/j.jadohealth.2007.02.015
[8] Didierlaurent AM, Morel S, Lockman L et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol 2009;15:6186–6197. doi: 10.4049/jimmunol.0901474
[9] Day PM, Kines RC, Thompson CD et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010;16:260–270. doi: 10.1016/j.chom.2010.08.003
[10] Cameron RL, Ahmed S, Pollock KG. Adverse event monitoring of the human papillomavirus vaccines in Scotland. Intern Med J 2016;46:452–457. doi: 10.1111/imj.13005
[11] Brinth LS, Pors K, Theibel AC et al. Orthostatic intolerance and postural tachycardia syndrome as suspected adverse effects of vaccination against human papilloma virus. Vaccine 2015;33:2602–2605. doi: 10.1016/j.vaccine.2015.03.098
[12] Mølbak K, Hansen ND & Valentiner-Branth P. Pre-vaccination care-seeking in females reporting severe adverse reactions to HPV vaccine. A registry based case-control study. PLoS One 2016;11:e0162520. doi: 10.1371/journal.pone.0162520
[13] European Medicines Agency (2016) Human papillomavirus vaccines. Available at: http://www.ema.europa.eu/ema/index.jsp%3Fcurl%3Dpages/medicines/human/referrals/Human_papillomavirus_vaccines/human_referral_prac_000053.jsp%26mid%3DWC0b01ac05805c516f (accessed March 2017)
[14] Potts A, Sinka K, Love J et al. High uptake of HPV immunisation in Scotland – perspectives on maximising uptake. Eurosurveillance 2013;18:20593. doi: 10.2807/1560-7917.ES2013.18.39.20593
[15] King EM, Gilson R, Beddows S et al. Human papillomavirus DNA in men who have sex with men: type-specific prevalence, risk factors and implications for vaccination strategies. Br J Cancer 2015;112:1585–1593. doi: 10.1038/bjc.2015.90
[16] Wakeham K & Kavanagh K. The burden of HPV-associated anogenital cancers. Curr Oncol Rep 2014;16:402. doi: 10.1002/ijc.30523
[17] Steinau M, Saraiya M, Goodman T et al. Human papillomavirus prevalence in oropharyngeal cancer before vaccine introduction, United States. Emerg Infect Dis 2014;20:822–828. doi: 10.3201/eid2005.131311
[18] Pinto LA, Kemp TJ, Torres BN et al. Quadrivalent human papillomavirus (HPV) vaccine induces hpv-specific antibodies in the oral cavity: results from the Mid-Adult Male Vaccine Trial. J Infect Dis 2016;214:1276–1283. doi: 10.1093/infdis/jiw359
[19] Scottish government (2016) HPV vaccine programme. Available at: http://news.gov.scot/news/hpv-vaccination-programme (accessed March 2017)
[20] Public Health England (2016) HPV vaccination pilot for men who have sex with men. Available at: https://www.gov.uk/government/publications/hpv-vaccination-pilot-for-men-who-have-sex-with-men-msm (accessed March 2017)
[21] Brotherton JML, Fridman M, May CL et al. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011;377:2085–2092. doi: 10.1016/S0140-6736(11)60551-5
[22] Paavonen J, Naud P, Salmeron J et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and pre-cancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4
[23] Cameron RL, Kavanagh K, Pan J et al. Human papillomavirus prevalence and herd immunity after introduction of vaccination program, Scotland, 2009-2013. Emerg Infect Dis 2016;22:56–64. doi: 10.3201/eid2201.150736
[24] Tabrizi SN, Brotherton JM, Kaldor JM et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis 2014;14:958–966. doi: 10.1016/S1473-3099(14)70841-2
[25] Baldur-Felskov B, Dehlendorff C, Munk C et al. Early impact of human papillomavirus vaccination on cervical neoplasia – nationwide follow-up of young Danish women. J Natl Cancer Inst 2014;106, djt460. doi: 10.1093/jnci/djt460
[26] Ali H, Donovan B, Wand H et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ 2013;346,f2032. doi: 10.1136/bmj.f2032
[27] Korostil IA, Ali H, Guy RJ et al. Near elimination of genital warts in Australia predicted with extension of human papillomavirus vaccination to males. Sex Transm Dis 2013;40:833–835. doi: 10.1097/OLQ.0000000000000030
[28] Palmer TJ, McFadden M, Pollock KG et al. HPV immunisation and cervical screening–confirmation of changed performance of cytology as a screening test in immunised women: a retrospective population-based cohort study. Br J Cancer 2016;114:582–589. doi: 10.1038/bjc.2015.474
[29] Cruickshank M, Pan J, Cotton SC et al. Reduction in colposcopy workload and associated clinical activity following HPV vaccination programme in Scotland: an ecological study. BJOG 2017. doi: 10.1111/1471-0528.14562
[30] Smith SP, Baxendale HE & Sterling JC. Clearance of recalcitrant warts in idiopathic immune deficiency following administration of the quadrivalent human papillomavirus vaccine. Clin Exp Dermatol 2017;Jan 10. doi: 10.1111/ced.13038
[31] Gillison ML, Chaturvedi AK, Anderson WF et al. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 2015;10:3235–3242. doi: 10.1200/JCO.2015.61.6995