Eye of Science / Science Photo Library
Pneumonia is usually caused by bacterial or viral infection, resulting in inflammation of the lungs (see Figure 1). Pneumonias represent a major medical and economic problem because they are the most frequent causes of morbidity and mortality worldwide[1]
, particularly in certain patient groups, such as people who are immunocompromised, children and older adults[2],[3]
.
Pneumonia can be broadly defined as community-acquired (acquired outside hospital or healthcare facilities) and hospital-acquired (acquired after 48 hours of admission to hospital)[1]
. The former can be caused by viruses; atypical bacteria (bacteria that are neither Gram-negative nor Gram-positive), including Mycoplasma pneumoniae, Chlamydophila pneumoniae and Legionella pneumophila; and fungi. Hospital-acquired pneumonia (HAP), although acquired in hospital, is considered distinct from pneumonia associated with mechanical ventilation, which is known as ventilator-associated pneumonia (VAP)[1]
.
An ever-increasing ageing population means that a greater proportion of individuals will be treated for respiratory tract infections, thus increasing the societal burden of this disease[4],[5]
. HAP increases the length of in-patient hospital stay and has been estimated to be the ‘attributable cause’ of death in between a third and a half of patients who are diagnosed with it.
This article will focus on the diagnosis and management of HAP episodes in older adults. It aims to support pharmacists and healthcare professionals in appropriately diagnosing and managing the condition.
Aetiology and epidemiology
The spectrum of organisms known to cause HAP and other respiratory infections is broad and constantly increasing. New pathogens are constantly being identified. However, it is frequently difficult for healthcare professionals to identify the causative organism for the pneumonia, as the patient’s immune response can be altered by medicines and other diseases[5]
. Even with a full range of microbiologic tests, the causative organisms can currently only be identified in around 30–70% of cases[5]
.
Viral and fungal aetiologies are rarer, but incidents for each can be relatively high in certain circumstances, such as in an influenza outbreak[6]
.
Bacteria, such as Streptococcus pneumoniae or Haemophilus influenzae, are the most common cause of HAP[2],[5],[7]
. Pseudomonas aeruginosa, Klebsiella spp., Enterobacteriaceae,
Escherichia coli, Serratia marcescens and Proteus spp. are common causes of HAP as length of hospital stay increases. HAP is estimated to increase hospital stays by seven to nine days. In addition, as length of hospital stay increases, it is more likely for multi-drug resistant (MDR) organisms to become the cause of the infection; these have increasingly been associated with a higher mortality[5]
. Furthermore, it has been reported that 5–10 per 1000 patients admitted to hospital develop HAP[2]
.
Risk factors
Previous intravenous antibiotic use (defined as antibiotic use within 90 days) is a significant risk factor for HAP caused by either methicillin-resistant Staphylococcus aureus (MRSA) or P. aeruginosa
[1]
. Additional risk factors for P. aeruginosa HAP include a history of chronic obstructive pulmonary disease (COPD), patients chronically colonised with P. aeruginosa, and prior use of carbapenems, broad spectrum cephalosporins or fluoroquinolones.
Ageing results in reduced lung elasticity and respiratory muscle strength (both inspiratory and expiratory); therefore, older patients may struggle to effectively clear their airways making them more prone to respiratory infections. The increase in co-morbidities in older people also adds to host defence impairment. Additionally, functional dependence, cognitive impairment, malnutrition and hypoalbuminaemia add to the risk factors for developing HAP. The role of silent aspiration is increasingly recognised in the pathogenesis of lower respiratory tract infection in older patients[2],[8],[9],[10],[11],[12]
and should be included in differential diagnoses.
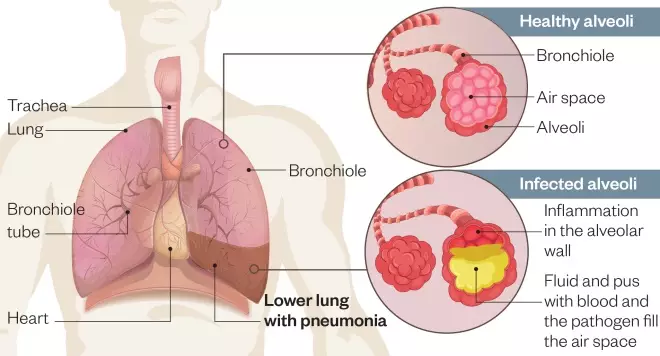
Figure 1: Pathogenesis of lower respiratory tract infection
Source: Shutterstock.com / MAG
Inflammation mainly affects the small air sacs, or alveoli, causing them to fill with fluid, pus and blood
Further risk factors contributing to the development of HAP[8]
can be found in Box 1: ‘Risk factors for hospital-acquired pneumonia’.
Box 1: Risk factors for hospital-acquired pneumonia (HAP)
- Patients who are immunocompromised (e.g. people living with HIV or having chemotherapy);
- Patients with co-morbidities (e.g. people with asthma or cystic fibrosis);
- Older people;
- People with previous hospital admissions;
- People on other medications (e.g. previous antibiotic exposure leading to selection pressure for resistant organisms; antacids and histamine2 -blockers, resulting in a rise of the gastric pH and an increase in gastric Gram-negative bacterial counts. This gastric reservoir for Gram-negative organisms frequently serves as a seed point for pharyngeal colonisation, and nasogastric tubes — which are present in many severely ill patients — may provide a pathway for the bacteria to migrate);
- Other hospital patients and staff with poor infection control/hand hygiene (e.g. bacteria that are transmitted to patients by healthcare workers’ hands).
Source: Centers for Disease Control and Prevention. https://www.cdc.gov/mmwr/preview/mmwrhtml/00045365.htm
Diagnosis
Symptoms
There is considerable variation in the clinical presentation of HAP, rendering distinction from other infections difficult (see Box 2: ‘Common symptoms of pneumonia’). The difficulties in making an accurate diagnosis has decreased the threshold for treatment of HAP, especially in older patients, owing to the high mortality rates associated with the condition[2]
.
Classic symptoms include fever, blood leucocytosis[2]
, leucopenia, malaise, cough, sputum production and chest pain. However, in practice, these symptoms may not necessarily be caused by pneumonia[2],[5],[10]
. Many disorders can mimic pneumonia, meaning that several differentials must be considered and potentially excluded, including pulmonary infarction, pulmonary oedema, pulmonary haemorrhage, pulmonary vasculitis, malignancy, iatrogenic lung shadowing and pre-existing lung disease[13],[14],[15]
.
Generally, the older and more debilitated the patient, the more likely the classic pneumonia syndrome will be incompletely expressed[2],[5],[16],[17]
. Decreased breath sounds, crackles and egophony (increased resonance of voice sounds) are poor diagnostic indicators; these must be combined with assessing vital sign abnormalities if specificity is to be improved. Fever is less commonly observed because many patients may be on regular agents with an antipyretic effect. Clinical observations such as confusion, delirium, tachypnoea (abnormally rapid breathing) and tachycardia are common and may be the only signs a patient exhibits[11]
.
Box 2: Common symptoms of pneumonia
- Cough;
- Difficulty breathing;
- Rapid heartbeat;
- Fever;
- Sweating and shivering;
- Loss of appetite;
- Chest pain.
Source: NHS Choices https://www.nhs.uk/conditions/pneumonia/
Biomarkers
Common point-of-care tests used to diagnose the presence of bacterial infections, and whether to initiate antibiotic therapy, include white cell count and C-reactive protein. Procalcitonin (PCT) is emerging as a promising diagnostic biomarker because it is released into the bloodstream during bacterial infection; therefore, high levels can indicate a severe bacterial infection, whereas low levels can indicate a viral infection. Recent Infectious Diseases Society of America and the American Thoracic Society guidelines recommend using clinical criteria alone, rather than using serum PCT plus clinical criteria, to decide whether or not to initiate antibiotic therapy[1]
. At present, there is not enough evidence to support the addition of procalcitonin testing to standard clinical practice in the NHS[18]
.
The Clinical Pulmonary Infection Score
The Clinical Pulmonary Infection Score (CPIS) is based on six criteria: fever, leucocytosis, positive sputum culture, worsening chest X-ray, oxygenation [PaO2/FiO2] and semi-quantitative cultures of tracheal aspirates, with or without Gram stain. A prospective cohort study investigated the utility of a modified CPIS in the diagnosis of HAP. Conventional clinical diagnosis was found to be inaccurate (sensitivity 50%, specificity 58%) but the CPIS was only slightly more accurate (sensitivity 60%, specificity 59%)[19]
. CPIS may be useful for selecting patients for short-course therapy and monitoring response to treatment. However, further data are required.
Imaging
A chest X-ray is an important examination in all patients suspected of having an infection. It should be carried out to confirm or exclude the presence of pulmonary abnormalities[14],[20],[21]
in addition to systemic symptoms to aid in the diagnosis. It is widely available, inexpensive and has a low radiation burden, fuelling its popularity for use in clinical investigation. It aids to narrow the differential diagnosis, evaluate complications, helps to direct additional diagnostic measures and serves as an ideal tool for follow-up examinations. Comparison with previous X-rays is useful to determine the presence of pneumonia. If follow-up exams are performed, it is important to consider the time period in which a pneumonic infiltrate may resolve. As a general rule, most pneumonias regress within 10–21 days. However, complete resolution may take up to six months, especially in patients with underlying lung disease (e.g. patients with COPD, immunocompromised individuals and older patients)[22],[23]
. However, the accuracy of chest X-rays can be limited in hospitalised individuals who have comorbidities; radiologic diagnosis of CAP is relatively straightforward in comparison[20]
.
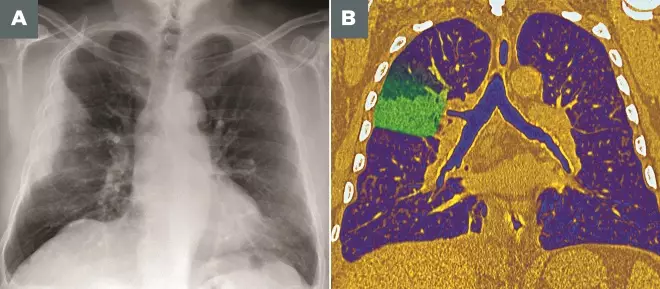
Photoguide: Imaging techniques for diagnosing pneumonia
Source: Pr Michel Brauner/ISM / Science Photo Library
A: Frontal X-ray of the lungs of a 53-year-old man, showing a partial lesion of the upper lobe of the right lung (upper left) due to bacterial pneumonia
B: Coloured frontal computed tomography (CT) scan of a section through the chest of a 60-year-old male patient with pneumococcal pneumonia affecting the upper lobe of the right lung (left)
It should be noted that the quality of the X-ray plays a critical role in the management of patients with suspected pneumonia[20]
. Older patients may not be able to inspirate/expirate to full capacity or remain in the necessary position required to ascertain a clear picture. Although some variation exists regarding the timeframe between the onset of clinical symptoms and the development of a radiographically visible abnormality, the vast majority of infiltrates appear within 12 hours[14]
. Hence, patients who develop symptoms of a pulmonary infection in a hospital setting may be seen within hours after the onset of clinical symptoms and a visible radiographic abnormality may not have developed; in immunocompromised patients the appearance of a detectable radiographic abnormality may be delayed[24],[25]
. It is important to note that in this group of patients, radiographic patterns change with immunologic status.
Imaging examinations should always be interpreted with a knowledge of how symptomatic the patient is, the degree of dyspnoea, the level of impairment of the carbon monoxide diffusing capacity of the lung, the CD4+ cell count, the presence of fever, if there is a cough (and whether it is productive), and the chronicity of symptoms[2]
.
Radiographically, patchy bronchopneumonia is the most common finding for HAP. Aspiration pneumonia is always an alternative diagnosis and should be suspected if pneumonia is present bilaterally in the dependent or posterior portions of the lungs[26]
.
In the absence of clinical information, radiologists cannot reliably distinguish between pneumonia and other pulmonary processes[27]
. Clinical information can greatly enhance the accuracy of a radiological diagnosis.
Pneumonia may exist without the typical appearance of a pulmonary infiltrate on the chest X-ray. Computerised tomography (CT) is more sensitive in the detection of subtle abnormalities and may show findings suggestive of pneumonia earlier than plain chest X-rays[20]
. CT and invasive diagnostic procedures should be considered in cases of treatment failure or where there are complexities. Computed immunities are reserved for unclear cases, particularly when chest X-rays are normal in patients with a high level of clinical suspicion of pneumonia, and in immunocompromised patients, in whom early diagnosis of pneumonia is important to determine a positive outcome.
Culture and invasive procedures
Sputum secretions are extremely difficult to obtain. They are often too poor quality to be used for culture, and are frequently contaminated by upper respiratory tract flora[28],[29]
. Therefore, not all organisms isolated from sputum secretions should be regarded as pathogens that necessarily require therapy; they should be interpreted and treated using the full clinical picture and, if necessary, in conjunction with consultant microbiologists. Older patients are generally unable to cough or expectorate, and so produce little or no sputum. Therefore, this approach is essentially ineffective and not cost-effective[5]
. Although isolation of organisms is helpful to guide treatment, it is not essential for diagnosis of HAP.
Further investigations, such as fine needle aspiration, should only be considered for patients who have not responded to initial treatment, in order to identify the causative organism for appropriate treatment selection[30],[31]
.
The use of invasive procedures, such as bronchoscopy to identify the potential causative agent, are frequently limited and may carry a high risk, especially in older patients, those that are immunocompromised and/or have coagulation disorders[5]
.
Treatment
Definitive diagnosis in HAP is difficult. The older and more infirm the patient, the more likely therapeutic difficulties will be encountered[5]
. Functional status, duration of hospitalisation, and concomitant medical illness are additional factors adding to the complexity and difficulty in treating these patients. If a positive microbiological result is attained, it may not influence outcome or change in therapy, thus raising the question of cost-effectiveness and exposure of risk to patients from procedures used to obtain samples. This has led to widespread empirical treatment[2],[5]
.
Empiric therapy should be based on local resistance patterns and recommendations. There are a number of antibiotics that are no longer regarded as appropriate empirical therapy for patients with HAP, because of dramatic changes in the nature and susceptibility patterns of the pathogens recognised in most UK hospitals[2]
. Empirical antibiotic therapy for patients with HAP should also be based on duration of hospital stay, host factors, co-morbidities and recent administration of antibiotic therapy. Similarly, definitive therapy should be determined by culture and susceptibility test results, if possible, but treatment should not be delayed because this has been associated with increased morbidity and mortality rates[32],[33]
.
There are many limitations in the quality of the available evidence to assist in approaches to antimicrobial selection in HAP. Few studies have compared more than two therapeutic options. Where possible, monotherapy should be used. This will aid in limiting the adverse drug reactions and toxicity that patients may experience. Combination therapy is advocated where activity against resistant organisms is required.
Risk factors should be taken into account when deciding which antibiotics to initiate. For patients being treated empirically for HAP, antibiotics with activity against S. aureus, such as piperacillin/tazobactam, ceftazidime or meropenem, should be prescribed. If risk factors for MRSA exist, the addition of teicoplanin or vancomycin is recommended. If risk factors for P. aeruginosa exist, antibiotics that have activity against these organisms should be prescribed. Specific patient factors, such as allergy status, should also be taken in account when deciding on a regimen.
Renal function must also be considered because many antibiotics are cleared renally. Dose adjustments may be required depending on the degree of renal insufficiency. Local guidelines, expertise and specific summary of product characteristics should be consulted for advice regarding dose and frequency.
Commonly, durations of between five and seven days have been used to treat infections. Increased use and increased treatment durations of antibiotics are known to add selection pressure for bacteria to develop resistance[4],[5],[34]
. National Institute for Health and Care Excellence (NICE) guidelines recommend a 5‑ to 10‑day antibiotic therapy course for patients with HAP[7]
. It also recommends that antibiotic therapy should be offered as soon as possible after diagnosis, and certainly within four hours[7]
.
However, patients should be treated for as long as necessary to resolve the infection. It is not necessary to follow patients radiographically when the clinical course indicates successful treatment. Where treatment failure is indicated, this may be as a result of a complication (e.g. sepsis), incorrect diagnosis or treatment of the wrong pathogen.
Initial treatment for HAP is generally parenteral. Patients should be switched to oral alternatives when they are improving clinically. Local guidelines and expertise (i.e. consultant microbiologist, infectious disease physician and antimicrobial pharmacist) should be consulted regarding the appropriate oral agent(s) to switch patients to. This will facilitate discharge, decrease patient exposure to acquire a hospital-acquired infection or superinfection, and be economically advantageous[34]
.
Physiotherapy
Chest physiotherapy is commonly used in patients with HAP. It may be of value to patients and might be appropriate as a therapeutic adjunctive option. However, data are lacking on its efficacy.
Prevention
Infection control is important in preventing HAP, for example good hand hygiene for healthcare professionals can help prevent the transmission of MDR pathogens that cause HAP (see Box 3: ‘Hand hygiene’).
Box 3: Hand hygiene
Transient flora are the most common cause of healthcare-associated infections (HCAIs), and are acquired and spread by direct contact with patients or environmental surfaces. If transferred into susceptible sites, such as invasive devices (e.g. central venous and urinary catheter) or wounds, these organisms can cause life-threatening infections.

Source: Shutterstock.com
In 2009, the World Health Organization produced guidelines on hand hygiene in healthcare, which outlines ‘five moments’ to perform hand hygiene:
- Before touching a patient;
- Before a clean or aseptic procedure;
- After body fluid exposure risk;
- After touching a patient;
- After touching a patient’s surroundings (e.g. changing bed linen).
Evidence is emerging that hand hygiene compliance after contact with patient surroundings is generally very poor in hospitals, and healthcare workers underestimate the role of environmental surfaces in transmission of HCAIs. Clinical notes, paper prescriptions and observation charts are part of the patient’s environment and any contact with these should be followed by appropriate hand hygiene.
There is no compelling evidence to favour a particular hand-washing agent (e.g. liquid soap, antiseptics) over others, even though their microbiological efficacy varies. Alcohol-based hand rubs are recommended by most national and international guidelines because of their ease of use and availability at the point of care. Alcohol hand rubs are suitable for use except when hands are visibly soiled or potentially contaminated with body fluids, or when caring for patients with vomiting or diarrhoeal illness. In these instances, soap and water should be used instead.
To wash hands correctly, both the hands and wrists need to be fully exposed to the product, and therefore should be free from jewellery and long-sleeved clothing (bare below elbow). Non-analytical studies and case reports have demonstrated that ties, rings and false nails worn by healthcare workers can be colonised with potentially harmful microorganisms, and can impede performing effective hand hygiene.
When using alcohol gel, hands should be free of dirt and organic material and the gel solution must come into contact with all surfaces of the hand; the hand should be rubbed vigorously until the solution has evaporated.
When washing hands with a liquid soap, the solution should come into contact with all surfaces of the hand and the hands should be rubbed together for a minimum of 10–15 seconds. Particular attention should be paid to the tips of the fingers, the thumbs and the areas between the fingers. Hands should be thoroughly rinsed and then dried with a good-quality paper towel.
Sources:
Centre for Disease Control and Prevention. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA hand hygiene task force. Morbidity and Mortality Weekly Report. 2002;51:2–6.
World Health Organization. Guidelines on hand hygiene in healthcare. 2009.
FitzGerald G, Moore G & Wilson APR. Hand hygiene after touching a patient’s surrounding: the opportunities most commonly missed. J Hosp Infect 2013;84(1):27–31.
Loveday HP, Wilson JA, Pratt RJ et al. EPIC3: National evidence-based guidelines for prevention healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2014;86(S1):S1–S70.
National Clinical Guideline Centre. Infection: prevention and control of healthcare-associated infections in primary and community care: partial update of NICE Clinical Guideline 2. NICE Clinical Guidelines, No. 139. London: Royal College of Physicians, 2012.
Summary
The diagnosis of HAP in older people is difficult, and the criteria used for surveillance have been based on clinical findings of fever, cough, and the development of purulent sputum in combination with a new or progressive infiltrate on the chest X-ray. Each patient should be assessed individually to ascertain the most likely cause, taking into account the patient’s condition and history. Therapy should be led by local guidelines, resistant patterns and patient factors.
Financial and conflicts of interest disclosure:
The author has no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. No writing assistance was used in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Kalil AC, Metersky ML, Klompas M et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63(5):e61–111. doi: 10.1093/cid/ciw353
[2] Masterton RG, Galloway A, French G et al. Guidelines for the management of hospital-acquired pneumonia in the UK: report of the Working Party on Hospital-Acquired Pneumonia of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 2008;62:5–34. doi: 10.1093/jac/dkn162
[3] Koivula I, Sten M & Makela PH. Risk factors for pneumonia in the elderly. Am J Med 1994;96:313–320. doi: 10.1016/0002-9343(94)90060-4
[4] Bowton DL & Bass DA. Community-acquired pneumonia: the clinical dilemma. J Thorac Imaging 1991;3:1–5. doi: 10.1097/00005382-199107000-00003
[5] Fein A. Pneumonia in the elderly: overview of diagnostic and therapeutic approaches. Clin Infect Dis 1999;28:726–729. doi: 10.1086/515218
[6] Kapila R, Lintz DI, Tecson FT et al. A nosocomial outbreak of influenza A. Chest 1977;71(5):576–579. doi: 10.1378/chest.71.5.576
[7] National Institute for Health and Care Excellence (NICE). Clinical guideline [CG191]. Available at: https://www.nice.org.uk/guidance/cg191/documents/pneumonia-final-scope2 (accessed February 2018)
[8] Centers for Disease Control and Prevention. Guidelines for prevention of nosocomial pneumonia. 1997. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/00045365.htm (accessed February 2018)
[9] American Thoracic Society. Guidelines for the initial management of adults with community-acquired pneumonia: diagnosis, assessment of severity, and initial antimicrobial therapy. Am Rev Respir Dis 1993;148:1418–1426. doi: 10.1164/ajrccm/148.5.1418
[10] Chastre J, Fagon JY, Soler P et al. Diagnosis of nosocomial bacterial pneumonia in intubated patients undergoing ventilation: comparison of the usefulness of bronchoalveolar lavage and the protected specimen brush. Am J Med 1988;85:499–506. doi: 10.1016/S0002-9343(88)80085-8
[11] Levy M, Dromer F, Brion N et al. Community-acquired pneumonia: importance of initial noninvasive bacteriologic and radiolographic investigations. Chest 1988;92:43–48. doi: 10.1378/chest.93.1.43
[12] Spencer H (1985) Pathology of the lung, 4th edn. Pergamon Press, Oxford, UK
[13] Lipchik RJ & Kuzo RS. Nosocomial pneumonia. Radiol Clin North Am 1996;34:47–58. PMID: 8539353
[14] Franquet T. Imaging of pneumonia: trends and algorithms. Eur Respir J 2001;18:196–208. doi: 10.1183/09031936.01.00213501
[15] Strain DS, Kinasewitz GT, Vereen LE & George RB. Value of routine daily chest X-rays in the medical intensive care unit. Crit Care Med 1985;13:534–536. doi: 10.1097/00003246-198507000-00004
[16] Fein A & Niederman M. Severe pneumonia in the elderly. Clin Geriatr Med 1994;10:121–143. PMID: 8168019
[17] Metlay JP, Kapoor WN & Fine MJ. Does this patient have community acquired pneumonia? J Am Med Assoc 1997;278:1440–1441. PMID: 9356004
[18] National Institute for Health and Care Excellence (NICE). Available at: https://www.nice.org.uk/researchrecommendation/use-of-biomarkers-to-diagnose-and-initiate-treatment-what-is-the-clinical-and-cost-effectiveness-of-procalcitonin-pct-point-of-care-tests-at-initial-triage-for-diagnosis-of-serious-infection-and-the-initiation-of-appropriate-antibiotic-therapy (accessed February 2018)
[19] West M, Boulanger BR, Fogarty C et al. Levofloxacin compared with imipenem/cilastatin followed by ciprofloxacin in adult patients with nosocomial pneumonia: a multicenter, prospective, randomized, open-label study. Clin Ther 2003;25:485–506. doi: 10.1016/S0149-2918(03)80091-7
[20] Herold CJ & Sailer JG. Community-acquired and nosocomial pneumonia. Eur Radiol Suppl 2004;14:E2. doi: 10.1007/s00330-003-2162-7
[21] Bruns AH, Oosterheert JJ, El Moussaoui R et al. Pneumonia recovery: discrepancies in perspectives of the radiologist, physician and patient. J Gen Intern Med 2010;25(3):203–206. doi: 10.1007/s11606-009-1182-7
[22] Mittl RL, Schwab RJ, Duchin JS et al. Radiographic resolution of community-acquired pneumonia. Am J Respir Crit Care Med 1994;149:630–635. doi: 10.1164/ajrccm.149.3.8118630
[23] Israel HL, Weiss W, Eisenberg GM et al. Delayed resolution of pneumonias. Med Clin North Am 1956;40:1291–1303. doi: 10.1016/S0025-7125(16)34506-0
[24] Zornoza J, Goldman AM, Wallace S et al. Radiologic features of Gram-negative pneumonias in the neutropenic patient. Am J Roentgenol 1976;127:989–996. doi: 10.2214/ajr.127.6.989
[25] Donowitz GR, Harman C, Pope T et al. The role of the chest roentgenogram in febrile neutropenic patients. Arch Intern Med 1991;151:701–704. doi: 10.1001/archinte.1991.00400040053012
[26] Strain DS, Kinasewitz GT, Vereen LE & George RB. Value of routine daily chest X-rays in the medical intensive care unit. Crit Care Med 1985;13:534–536. doi: 10.1097/00003246-198507000-00004
[27] Primack SL & Müller NL. HRCT in acute diffuse lung disease in the immunocompromised patient. Radiol Clin North Am 1994;32:731–744. PMID: 8022977
[28] Bartlett RC & Melnick A. Usefulness of Gram stain and routine and quantitative culture of sputum in patients with and without acute respiratory infection. Conn Med 1970;34:347–351. PMID: 4194107
[29] Woodhead MA, MacFarlane JT, McCracken JS et al. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet 1987;21:671–674. doi: 10.1016/S0140-6736(87)90430-2
[30] Conces DJ, Clark SA, Tarver RD & Schwenk CR. Transthoracic-aspiration needle biopsy: value in the diagnosis of pulmonary infections. Am J Roentgenol 1989;152(1):31–34. doi: 10.2214/ajr.152.1.31
[31] Sanchez-Nieto JM, Torres A, Garcia-Corboda F et al. Impact of invasive and noninvasive quantitative culture sampling on outcome of ventilator associated pneumonia. Am J Resp Crit Care Med 1998;157:371–376. doi: 10.1164/ajrccm.157.2.97-02039
[32] Luna CM, Vujacich P, Niederman MS et al. Impact of BAL data on the therapy and outcome of ventilator associated pneumonia. Chest 1997;111:676–685. doi: 10.1378/chest.111.3.676
[33] Iregui M, Ward S, Sherman G et al. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator associated pneumonia. Chest 2002;122:262–268. doi: 10.1378/chest.122.1.262
[34] Jethwa S. Principles of initiating antimicrobial therapy and empiric prescribing. Clin Pharm 2016;8(8). doi: 10.1211/CP.2016.20201507