Science Photo Library
Summary
Despite a better understanding of the pathogenesis and treatment of peptic ulcer, Helicobacter pylori infections and treatment remain an unsolved problem with around half of the world’s population still infected. The prevalence ranges from >80% in many developing countries to around 10% in developed western countries.
H. pylori gastritis is fundamentally an infectious disease and for more than 100 years gastritis was known to be closely associated with peptic ulcer and gastric cancer. Despite H. pylori being identified as a class I (definite) carcinogen in 1994, its role in gastric cancer remained under appreciated until recently, when worldwide programmes to eradicate H. pylori begun to be seriously considered as a method to eradicate gastric cancer.
Therapy for H. pylori is now recommended for all patients with the infection, irrespective of the presence or absence of symptoms or complications. The focus is now on who to test, rather than who to treat, and the decision to treat does not guarantee treatment success. Successful therapy of H. pylori is based on providing an acceptable regimen to which the organism is susceptible. The inability to obtain susceptibility testing is one of the reasons why new drug combinations for H. pylori have largely been developed by trial and error, often using arbitrary doses, dosing intervals and duration of therapy. This approach has led to many new regimens that initially proved successful in one population and were touted as ‘superior’, and were subsequently shown to be generally ineffective.
With few exceptions, effective H. pylori therapy currently requires two or more antimicrobials, an antisecretory drug to reduce gastric secretion, and a duration of 14 days. Increasing the gastric pH improves the ability of antimicrobials to function and encourages H. pylori to replicate, which in turn makes the cells susceptible to antimicrobials, such as beta-lactams, that only act during replication. Reliable cure rates of 95% or greater are now possible with a variety of different regimens, provided that antimicrobial resistance is not present.
Appreciation of the importance of Helicobacter pylori infections has evolved since the first culture of the organism in the early 1980s. The initial focus was on the relation of H. pylori to peptic ulcer disease, which at the time was a major clinical problem responsible for much morbidity, many hospitalisations and thousands of operations annually. In the 1970s, there were an estimated 500,000 new ulcer cases in the United States each year, with more than 400,000 hospitalisations and more than 4 million hospital days devoted to the treatment of peptic ulcers. In addition, there were approximately 140,000 ulcer operations each year[1]
.
In the 1980s, following the culture of H. pylori, the data to prove that the infection was aetiologically-related to peptic ulcers were obtained, accurate diagnostic methods were developed and popularised, and antimicrobial regimens that would reliably cure the infection were identified. The introduction of H. pylori therapy changed peptic ulcer disease from a lifelong problem associated with recurring symptoms (an approximately 25% risk of a major complication such as a gastrointestinal haemorrhage, curable only by surgery), into a complication of an infectious disease that was both preventable and curable following a short course of antibiotics.
Despite a better understanding of the pathogenesis and treatment of peptic ulcer, H. pylori infections and treatment remain an unsolved problem: around half of the world’s population is still infected. The prevalence ranges from >80% in many developing countries to around 10% in developed western countries. The prevalence still remains high in low income populations and immigrants from developing countries. H. pylori infection is aetiologically-related to a variety of diseases other than peptic ulcer, including gastric adenocarcinoma, gastric lymphoma, gastric atrophy with vitamin B12 deficiency (pernicious anaemia), iron deficiency anaemia, idiopathic thrombocytopaenia and non-ulcer dyspepsia[2]
.
H. pylori gastritis is fundamentally an infectious disease[2]
. For more than 100 years, gastritis was known to be closely associated with peptic ulcer and gastric cancer. Gastric cancer was the most common cause of cancer death in the first half of the 20th century and even now is one of the top four causes. Despite H. pylori being identified as a class I (definite) carcinogen in 1994, its role in gastric cancer remained under appreciated until recently[3]
, when worldwide programmes to eradicate H. pylori begun to be seriously considered as a method to eradicate gastric cancer[2]
. The publication of results of a randomised, double-blind, placebo-controlled, phase III trial of an anti-H. pylori vaccine has potentially opened the way to prevent transmission of the infection in developing countries, where the infection is common but poor sanitation, poverty and other major health problems otherwise prevent implementation of antimicrobial-based eradication programmes[4]
.
Therapy for H. pylori is now recommended for all patients with the infection, irrespective of the presence or absence of symptoms or complications[2],
[3]
. The focus is now on who to test, rather than who to treat and the decision to treat does not guarantee treatment success. Successful therapy of infectious diseases is based on providing an acceptable regimen to which the organism is susceptible[3]
; H. pylori is no exception. Although almost all hospitals, clinics and GP surgeries have access to facilities for the identification and provision of antimicrobial susceptibility testing for important local pathogens, susceptibility testing for H. pylori remains difficult, or even impossible for clinicians to obtain. The inability to obtain susceptibility testing is one of the reasons why new drug combinations for H. pylori have largely been developed by trial and error, often using arbitrary doses, dosing intervals and duration of therapy. This approach has led to many new regimens that initially proved successful in one population and were touted as ‘superior’, and were subsequently shown to be generally ineffective. Such regimens (e.g. sequential therapy) often received widespread use in populations with different patterns of antimicrobial resistance, where they produced unacceptably low cure rates[3]
. The failure to examine effectiveness in susceptible and resistant strains, and to recognise that what appeared to be superior results actually reflected differences in effectiveness in relation to specific antimicrobial resistance patterns, led to misinterpreted conclusions and many flawed meta-analyses that ultimately resulted in many thousands of patients receiving inadequate therapy and experiencing treatment failure[3],
[5]
.
Poor cure rates with regimens that reliably yield 95% or greater cure rates in adherent patients with susceptible strains are most often caused by the presence of antimicrobial resistance, poor adherence to the regimen, or both. As such, clinical trials that do not provide results linked with susceptibility and adherence are not interpretable and their results cannot be generalised (i.e. the results are specific to the population being studied). Most published studies and meta-analyses regarding H. pylori therapy fall into the category of ‘clinically uninterpretable’.
Useful H. pylori therapies
An optimal therapy is defined as one that will reliably cure at least 95% of infections in adherent patients with susceptible infections. An acceptable empiric therapy is one that will reliably cure at least 90% of infections in adherent patients. With few exceptions, effective H. pylori therapy currently requires two or more antimicrobials, an antisecretory drug to reduce gastric secretion, and a duration of 14 days. Increasing the gastric pH improves the ability of antimicrobials to function and encourages H. pylori to replicate, which in turn makes the cells susceptible to antimicrobials, such as beta-lactams, that only act during replication[6],
[7]
. Reliable cure rates of 95% or greater are now possible with a variety of different regimens, provided that antimicrobial resistance is not present (see ‘Recommended regimens for Helicobacter pylori therapy’)[3],
[8],
[9],
[10]
.
| Recommended regimens for Helicobacter pylori therapy | |
|---|---|
| PPI: proton-pump inhibitor. *PPIs generally not affected by CYP2C19 genotype are preferred (e.g. esomeprazole or rabeprazole). | |
| Treatment | Drugs, dosages and duration |
| Empiric therapies | When susceptibility testing is not available and the pattern of antimicrobial resistance in the regions is reasonably well known |
| Concomitant therapy | Amoxicillin (1g), clarithromycin (500mg) and tinidazole (500mg) or metronidazole (500mg) plus a PPI (40mg omeprazole* equivalent per dose) all given twice daily for 14 days. |
| Bismuth quadruple therapy | Bismuth subsalicylate or bismuth subcitrate two tablets and tetracycline hydrochloride (500mg) both four times daily with meals and at bedtime, plus metronidazole/tinidazole (500mg) three times daily with meals and a PPI twice daily for 14 days (40mg omeprazole equivalent per dose). Doxycycline cannot be substituted. |
| For prepackaged bismuth quadruple therapy | PYLERA for 14 days, add a PPI twice daily (40mg omeprazole equivalent per dose). |
| Salvage therapies | After at least two failures with different antimicrobials |
| Rifabutin triple therapy (best if susceptability based) | Rifabutin (150mg) daily, amoxicillin (1.5g) every eight hours and pantoprazole (80mg), or an equivalent PPI, every eight hours for 14 days, consider adding bismuth two tablets twice daily. |
| High dose PPI-amoxicillin dual therapy | PPI (e.g. rabeprazole 20mg, esomeprazole 40mg plus amoxicillin 750mg all four times daily at approximately six-hour intervals for 14 days) plus no acid drinks (e.g. soft drinks) or foods. |
| Susceptibility-based therapies | Based on known susceptibility testing or low resistance populations |
| Clarithromycin triple therapy — when H. pylori infection is known to be susceptible to clarithromycin | Amoxicillin (1g) and clarithromycin (500mg), plus a PPI all given twice daily for 14 days (40mg omeprazole equivalent per dose). |
| Metronidazole therapy — when H. pylori is known to be susceptible to nitroimidazoles | Amoxicillin (1g) and tinidazole (500mg) or metronidazole (500mg) plus a PPI all given twice daily for 14 days (40mg omeprazole equivalent per dose). |
| Clarithromycin-metronidazole triple therapy — when H. pylori is susceptible to both and in penicillin allergy | Clarithromycin (500mg) and tinidazole (500mg) or metronidazole (500mg), plus a PPI all given twice daily for 14 days (40mg omeprazole equivalent per dose). |
| Fluoroquinolone therapy — when H. pylori is known to be susceptible to fluoroquinolones | Fluoroquinolone (e.g. levofloxacin 500mg once daily), plus a PPI and amoxicillin 1g twice daily for 14 days. |
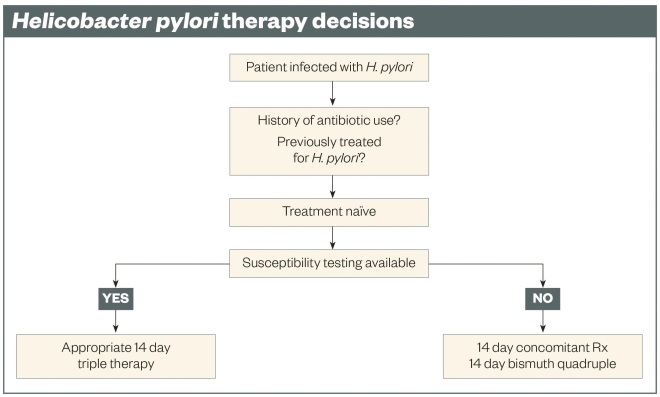
The best cure rates are achieved with double-dose proton pump inhibitor (PPI) therapy (e.g. omeprazole 40mg or equivalent) and with therapy duration of 14 days. Effective regimens can contain two, three, four or more drugs. Ideally, and for the best results, the choice of therapy should be susceptibility-based (see ‘Helicobacter pylori therapy decisions’). This can be achieved by culture testing, or for some antimicrobials, molecular testing[11]
. Population-based susceptibility testing allows empiric therapy but requires surveillance of the patterns of resistance, routinely carrying out cure confirmation tests, and collating a careful history of prior antimicrobial therapy. For susceptibility-based therapy, the most reliable and most tolerable regimens are three-drug regimens (triple therapies) given twice a day for 14 days (see ‘Recommended regimens for Helicobacter pylori therapy’). Four-drug regimens are often used as empiric therapy when susceptibility testing is not available and the pattern of antimicrobial resistance in the region is reasonably well known.
Triple therapies (susceptibility-based therapies)
What is known as classical triple therapy consists of a PPI and two antimicrobials, originally amoxicillin, clarithromycin or metronidazole, using the formula of a PPI, amoxicillin, and a third drug. For patients with penicillin allergy, the combination of PPI, clarithromycin and metronidazole is used. More recently, a new generation fluoroquinolone (e.g. levofloxacin) has been used: PPI, amoxicillin, levofloxacin triple therapy. Resistance to macrolides, nitroimidazole, and fluoroquinolones has been increasing directly in relation to the overall use of these drugs[12]
and frequently occurs following use of one of these antimicrobials, even for an indication other than the treatment of H. pylori. Cross-resistance within a group is also common (e.g. after any macrolide or fluoroquinolone). Therefore, most antimicrobials (with the exception of amoxicillin and tetracycline where resistance rarely occurs even after failed therapy) are effectively removed from the regimen for those with resistant strains: triple therapy is converted to a PPI plus amoxicillin.
For the individual patient, antimicrobial resistance results in unacceptably low cure rates. The cure rate for a population is calculated as the sum of those with susceptible infections (e.g. 97%) plus those with resistant infections (e.g. 0–40%)[3],
[10],
[13]
. The average cure rate for a population will generally fall below 90% when the prevalence of resistance is greater than 8–10%. Worldwide, the prevalence of resistance to clarithromycin, metronidazole and levofloxacin has achieved a level (e.g. over 15%), that precludes the empiric use of these regimens[3],
[10],
[13]
. Triple therapies are thus considered obsolete, except in a few populations, including Thailand and Northern Europe, and when used as susceptibility-based therapy.
Non-bismuth-containing four-drug therapies
On account of simplicity, cost and tolerance, there is a preference for appropriate triple therapy for infections known to be susceptible (see ‘Helicobacter pylori therapy decisions’). Increasing antimicrobial resistance has resulted in an increase in the number of antimicrobials that are combined to produce an empiric therapy. Two regimens containing a PPI, amoxicillin, clarithromycin and metronidazole were introduced in the late 1990s: concomitant therapy, when the four drugs are given together, and sequential therapy when given sequentially. Sequential therapy was originally designed as a ten-day therapy (e.g. a PPI and amoxicillin, followed by the PPI, clarithromycin, and metronidazole: 5+5 days) but it was later optimised with an improved cure rate by increasing the duration to 14 days (7+7 days). Sequential therapy is now obsolete because it is complicated and concomitant therapy will provide a superior, or at worst, equivalent result[3]
.
Concomitant therapy can be considered as functionally giving 14-day clarithromycin triple therapy and 14-day metronidazole triple therapy simultaneously. As such, it becomes ineffective only in the presence of dual metronidazole and clarithromycin resistance, which in most populations is rare. Consider a population with 30% metronidazole resistance and 15% clarithromycin resistance and where both triple and sequential therapy would produce poor cure rates. In that population, dual resistance would be less than 6% (e.g. 30% of 15% = 4.5%) and the therapy would achieve greater than 90% cure rates. The primary use for concomitant therapy is as an empiric therapy in regions where the prevalence of clarithromycin and metronidazole resistance is common but modest. Concomitant therapy is not a prudent choice for second-line therapy after treatment failure with a regimen containing either metronidazole or clarithromycin (see ‘What to do after one or more treatment failures’ and ‘Recommended regimens for Helicobacter pylori therapy’ for second-line therapy and salvage therapy options).
Bismuth-containing four-drug therapies
In western countries this regimen, often called bismuth quadruple therapy, consists of a PPI, bismuth subcitrate or subsalicylate, tetracycline and metronidazole[14]
. This was the first truly effective regimen and, when given at full dosage and for 14 days, metronidazole resistance can largely be overcome. In addition, tetracycline resistance is rare in most countries. The main disadvantage is the relatively high rate of adverse events (e.g. 50% of patients are expected to experience nausea or abdominal pain). However, with pre-therapy counselling, most patients will be able to complete the regimen (the failure rate to take the therapy is about 5%). Another disadvantage is that tetracycline has become difficult to obtain in many countries and doxycycline cannot be substituted as it typically fails when metronidazole resistance is present[14]
. The commercially available combined prepackaged product, Pylera, is unfortunately packaged for ten-day therapy, which is thought to be inferior to 14-day therapy when metronidazole resistance is present — metronidazole resistance is generally common worldwide. The primary indications for this regimen are the inability to take penicillins, presence of metronidazole resistance, and as a second-line therapy when susceptibility testing is not available. Metronidazole triple therapy would be preferred for metronidazole-susceptible infections.
Adjuvants
To date, adjuvants such as pronase, n-acetylcysteine, or probiotics have been inconsistently shown to improve efficacy of H. pylori therapies. Probiotics have been shown in some instances to reduce side effects but the exact details of doses and the probiotic regimen has not yet been satisfactorily defined[14],
[15]
. The cost is also considerable. More research is needed to identify which probiotics are helpful and to show that the additional cost brings an appropriate benefit.
Who to treat and which therapy to use in treatment-naïve patients
H. pylori gastritis is an infectious disease associated with progressive gastric damage and is a major aetiologic factor in peptic ulcer disease and gastric cancer. As such, the recommendation from the Kyoto Global consensus is that the infection should be eradicated unless there are compelling reasons not to[2]
. Currently, recommended effective therapies are all 14 days in duration and consist of a PPI and one or more antimicrobials. Ideally, choice of therapy should be based on the results of culture and susceptibility testing or molecular-based susceptibility testing. With those data, it is easy to choose a triple therapy that will reliably produce a very high cure rate (e.g. triple therapies in ‘Recommended regimens for Helicobacter pylori therapy’).
If susceptibility testing is not available, the regimen that works best locally should be used, with the choice being tempered by data obtained from the patient and their chart regarding prior antimicrobial use, drug allergies and intolerances, cost and drug availability. As noted above, it can generally be assumed that clarithromycin, levofloxacin, and metronidazole resistance are likely present and none of those drugs should be used as a triple therapy unless susceptibility has been confirmed. This restricts empiric therapies to four-drug regimens (see ‘Recommended regimens for Helicobacter pylori therapy’ and ‘Helicobacter pylori therapy decisions’). The two major choices are concomitant therapy and bismuth quadruple therapy (see ‘Recommended regimens for Helicobacter pylori therapy’). Generally, concomitant therapy is better tolerated and is usually the first choice. Inability to take amoxicillin would exclude all amoxicillin-containing therapies (unless desensitised), making bismuth quadruple therapy the first choice.
Test for cure
H. pylori eradication therapy should be followed by a test for cure. In most instances, a non-invasive test such as a urea breath test or monoclonal stool antigen test can be used to confirm cure. When endoscopy is required for other reasons, such as follow-up of a suspicious gastric ulcer, histology can be used to confirm if the therapy was successful. Serology generally remains positive and cannot be used to assess the results of therapy. Confirmation of cure should be delayed at least four weeks following the end of antimicrobial therapy (six weeks if the stool antigen test is used). Antibiotics, bismuth and PPIs all reduce the bacterial load and the test for cure should be delayed for at least two weeks if these are given after the end of eradication therapy to allow the organisms, if present, to return to a detectable level. If an antisecretory therapy is needed, a H2-receptor antagonist can be used, as they do not affect bacterial growth. Reinfection is rare and recurrence most often represents recrudescence of the original infection rather than reinfection from a new source.
What to do after one or more treatment failures
Whenever possible, the next regimen should be susceptibility-based. If testing is not available, one must take a careful history of prior antimicrobial use to try to identify a regimen that uses antimicrobials not used previously. Generally, if one used concomitant therapy, the next choice would be bismuth quadruple therapy and vice versa. For those regions where furazolidone is available, furazolidone-containing four-drug regimens are recommended because of the high rate of treatment success despite resistance to other antimicrobials[16],
[17]
. However, the best results will always be susceptibility-based. Rifabutin is used for treatment of tuberculosis and because of its low usage level, resistance is rare. A rifabutin-containing therapy that is reliably effective in different regions has not yet been identified. For dual PPI-amoxicillin therapy (see ‘The future’).
Patient education
Excellent results require the patient to adhere to the treatment schedule. Time spent on patient education is time well spent as treatment failure allows progression of the gastric mucosal damage, recurrence or development of peptic ulcer, an increased risk of gastric cancer and transmission of the disease to others[18],
[19],
[20]
. Treatment failure is expensive as it requires more tests, treatments, post-treatment evaluations and doctor visits (i.e. costly both in medical resources and time). The patient should fully understand the gains from successful H. pylori eradication and why they should stick with, and complete, the therapy despite inconvenient side effects. The regimen should be discussed in detail in relation to the drugs, the timing of drug administration and the anticipated side effects (e.g. strange taste in the mouth, black stool and nausea). A prescription without discussion and buy-in from the patient is a recipe for many failed treatment attempts.
The future
New antimicrobials are being developed as well as new antisecretory agents. Hopefully, these will simplify anti-H. pylori therapy. The new potassium-competitive acid blockers will theoretically allow more reliable pH control[21]
. If the pH can be maintained above six, it is theoretically possible to use only one antibiotic (e.g. amoxicillin) and cure most infections[6]
,[22]
. This has proven difficult or impossible with current PPIs, at least in western populations. An initial study of the potassium-competitive acid blocker vonoprazan in Japan used 20mg of vonoprazan twice daily, clarithromycin 200mg or 400mg twice daily, and amoxicillin 750mg twice daily for seven days[23]
. The therapy was equivalent to PPI-containing triple therapy with susceptible infections (97% cured). Those with clarithromycin resistance effectively received only vonoprazan and amoxicillin, and the cure rate with vonoprazan was only 82%. This suggests that longer duration, more frequent administration of amoxicillin, a higher dose of vonoprazan, or some combination of these variables, will be required to achieve acceptable results with vonoprazan-containing dual therapy[24]
. In addition to the new antimicrobials and new antisecretory agents being developed, a vaccine that prevents infection in children has been reported in China. H. pylori infections are typically acquired in childhood and, if confirmed, a vaccine would potentially allow for an H. pylori eradication programme in developing countries, which serve as a major reservoir of the infection[4]
.
David Y Graham, MD, is professor of medicine, molecular virology and microbiology, Baylor College of Medicine and Michael E DeBakey Veterans Affairs Medical Center, Houston, Texas, United States.
Support and conflict of interest disclosure: David Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grants DK56338, which funds the Texas Medical Center Digestive Diseases Center, and DK067366. The contents are solely the responsibility of the author and do not necessarily represent the official views of Veterans Affairs or the National Institute of Health. Graham is a paid consultant for RedHill Biopharma regarding novel H. pylori therapies and for Otsuka Pharmaceuticals regarding diagnostic testing. Graham has received royalties from Baylor College of Medicine patents covering materials related to 13C-urea breath test.
References
[1] Soll AH, Weinstein WM, Kurata J et al. Nonsteroidal anti-inflammatory drugs and peptic ulcer disease. Ann Intern Med 1991;114:307–319. doi:10.7326/0003-4819-114-4-307
[2] Sugano K, Tack J, Kuipers EJ et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64(9):1353–1367. doi:10.1136/gutjnl-2015-309252
[3] Graham DY. Helicobacter pylori update: Gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015;148(4):719–731. doi:10.1053/j.gastro.2015.01.040
[4] Zeng M, Mao XH, Li JX et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386(10002):1457–1464. doi:10.1016/s0140-6736(15)60310-5
[5] Gatta L, Vakil N, Vaira D et al. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ 2013;347:f4587. doi:10.1136/bmj.f4587
[6] Marcus EA, Inatomi N, Nagami GT et al. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther 2012;36(10):972–979. doi:10.1111/apt.12059
[7] Sachs G, Wen Y & Scott DR. Gastric infection by Helicobacter pylori. Curr Gastroenterol Rep 2009;11(6):455–461. doi:10.1007/s11894-009-0070-y
[8] Rimbara E, Fischbach LA & Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol 2011;8(2):79–88. doi:10.1038/nrgastro.2010.210
[9] Graham DY, Lee YC & Wu MS. Rational Helicobacter pylori therapy: Evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol 2014;12(2):177–186. doi:10.1016/j.cgh.2013.05.028
[10] Wu JY, Liou JM & Graham DY. Evidence-based recommendations for successful Helicobacter pylori treatment. Expert Rev Gastroenterol Hepatol 2014;8(1):21–28. doi:10.1586/17474124.2014.859522
[11] Megraud F, Benejat L, Ontsira Ngoyi EN et al. Molecular approaches to identify Helicobacter pylori antimicrobial resistance. Gastroenterol Clin North Am 2015;44(3):577–596. doi:10.1016/j.gtc.2015.05.002
[12] Megraud F, Coenen S, Versporten A et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013;62(1):34–42. doi:10.1136/gutjnl-2012-302254
[13] Liou JM, Chen CC, Chen MJ et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 2013;381(9862):205–213. doi:10.1016/s0140-6736(12)61579-7
[14] Graham DY, Lee SY. How to effectively use bismuth quadruple therapy: The good, the bad, and the ugly. Gastroenterol Clin North Am 2015;44(3):537–563. doi.org/10.1016/j.gtc.2015.05.003
[15] Graham DY. Editorial — avoiding unethical Helicobacter pylori clinical trials: susceptibility-based studies and probiotics as adjuvants. Helicobacter 2015;20(5):321–325. doi:10.1111/hel.12244
[16] Liang X, Xu X, Zheng Q et al. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol 2013;11(7):802–807. doi:10.1016/j.cgh.2013.01.008
[17] Lu H, Zhang W & Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepatol 2013;25(10):1134–1140. doi:10.1097/meg.0b013e3283633b57
[18] Al-Eidan FA, McElnay JC, Scott MG et al. Management of Helicobacter pylori eradication — the influence of structured counselling and follow-up. Br J Clin Pharmacol 2002;53(2):163–171. doi:10.1046/j.0306-5251.2001.01531.x
[19] Lee M, Kemp JA, Canning A et al. A randomized controlled trial of an enhanced patient compliance program for Helicobacter pylori therapy. Arch Intern Med 1999;159(19):2312–3216. doi:10.1001/archinte.159.19.2312
[20] O’Connor JP, Taneike I & O’Morain C. Improving compliance with Helicobacter pylori eradication therapy: when and how? Therap Adv Gastroenterol 2009;2(5):273–279. doi:10.1177/1756283x09337342
[21] Garnock-Jones KP. Vonoprazan: first global approval. Drugs 2015;75(4):439–443. doi:10.1007/s40265-015-0368-z
[22] Graham DY & Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol 2008;5(6):321–331. doi:10.1038/ncpgasthep1138
[23] Murakami K, Sakura Y, Shiino M et al. Tu1056 a phase 3, double-blind study of a triple therapy with TAK-438, amoxicillin, and clarithromycin as first line eradication of H. pylori and a triple therapy with TAK-438, amoxicillin, and metronidazole as second line eradication of H. pylori. Gastroenterology 2014;146(5):S-740. doi:10.1016/s0016-5085(14)62679-2
[24] Furuta T & Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori Infection. Gastroenterol Clin North Am 2010;39(3):465–480. doi:10.1016/j.gtc.2010.08.007