David Caulfield
Cancer learning ‘hub’
Pharmacists are playing an increasingly important role in supporting patients with cancer, working within multidisciplinary teams and improving outcomes. However, in a rapidly evolving field with numbers of new cancer medicines is increasing and the potential for adverse effects, it is now more important than ever for pharmacists to have a solid understanding of the principles of cancer biology, its diagnosis and approaches to treatment and prevention. This new collection of cancer content, brought to you in partnership with BeOne Medicines, provides access to educational resources that support professional development for improved patientChimeric antigen receptor T-cell treatment cycle
T-cells are a subset of lymphocytes that protect the body from pathogens and cancer cells. Unfortunately, cancerous cells have a range of mechanisms to avoid T-cell detection, such as downregulating the T-cell response if a tumour cell is detected and upregulating the number of inhibitory T-cells. However, CAR-T cells can detect and kill cancer cells. This is possible because a gene which confers antigen specificity, via a chimeric antigen receptor, is inserted into the T-cell during the manufacturing process. This focuses the T-cell on targeting and destroying specific cancer cells[7] . Figure 1 shows the process for manufacturing autologous CAR-T cells[8] . 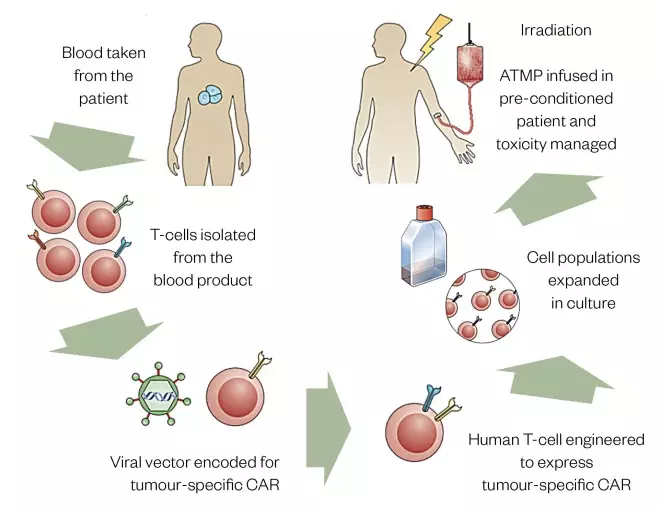
Figure 1: Autologous chimeric antigen receptor T-cell treatment cycle
Source: Adapted by Trakcel from Nat Rev Immunol [31]
ATMP: advanced therapy medicinal products; CAR: chimeric antigen receptor
Patient and product journeys
The patient journey begins when a patient who fulfils the eligibility criteria for CAR-T cells consents to receiving the treatment (see Figure 2). They then visit the apheresis centre, where they are attached to an apheresis machine for their peripheral blood mononuclear cells to be harvested. Apheresis usually takes 3.5–4.5 hours and is performed in accordance with the manufacturer’s apheresis manual, which may dictate a defined blood volume or an absolute T-cell count. Typically, 10–12L of blood volume is circulated, resulting in around 100mL of apheresis material generated. After a period of four to eight weeks, during which some bridging chemotherapy may be required to stabilise their disease, treatment continues with the administration of lymphodepleting chemotherapy to prepare the patient’s immune system for the CAR-T cells, which are subsequently infused. Product-associated toxicities may ensue, which require careful clinical management, potentially in the intensive care unit. All patients who receive this treatment should carry a patient informatioÂn card stating that they have received marketed CAR-T cells along with instructions to be followed in the event of an emergency. The product journey begins with the processing of the harvested apheresis material in the stem cell laboratory, if required by the manufacturer. The cells are packaged and shipped to the manufacturer, where the regulatory controls shift from the Human Tissue Authority (HTA), in the UK, to the local medicine’s authority, as the material is modified and becomes a medicine. When the products are manufactured outside of the EU, the cells must be exported in accordance with an HTA licence[9] . The bespoke medicinal product is frozen and shipped to the hospital where it is verified whether it has been received undamaged and labelled correctly. It should be stored under vapour phase liquid nitrogen, at temperatures below –120°C or –150°C, depending on the product, until it can be correctly thawed and administered. Specialist handling requirements are required and each commissioned centre has established optimal processes to allow this to occur. Figure 2 combines the patient and product journey, highlighting the complexity of the processes involved in the provision of CAR-T cell therapy and the stages at which pharmacy team involvement is recommended. The Pan-UK Pharmacy Working Group for ATMPs (a network of NHS hospital pharmacists working with ATMPs), in conjunction with the NHS Specialist Pharmacy Service, has produced checklists that help ensure that the major points at each stage are covered by local systems[10] . These checklists ensure that pharmacists provide consistent oversight of medicines and that products are handled by healthcare professionals with the appropriate skills and competencies, regardless of where they work.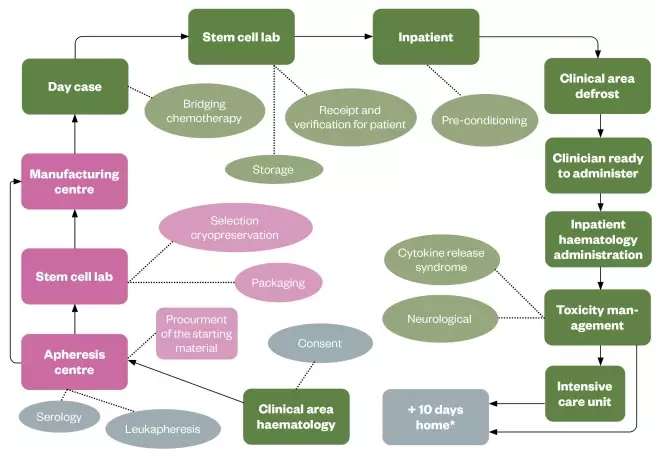
Figure 2: Pharmacy involvement in marketed chimeric antigen receptor T-cell journey
*For four weeks after treatment, patients must remain with a carer within one hour’s travel from the treating hospital in case of occurrence of cytokine release syndrome / immune effector cell associated neurotoxicity. Key: green: areas of pharmacist Involvement; grey: patient journey; pink: product journey
Therapeutic indications
Patients must receive lymphodepleting chemotherapy with fludarabine and cyclophosphamide before receiving CAR-T cell therapy. This process creates a favourable immune environment for CAR-T cells to expand (replicate) once they are infused[11] . A trained haematology pharmacist must screen the prescription and co-ordinate with the pharmacy aseptic unit, nurses and stem cell laboratory to ensure safe and accurate timing of chemotherapy prior to CAR-T cell infusion.Why cellular medicines are important
Before CAR-T cell therapy was approved for use in the EU in 2018, salvage chemotherapy followed by high-dose therapy and autologous stem-cell transplantation (ASCT) was the standard treatment for relapsed DLBCL. Nearly 40% of relapsed or refractory patients did not obtain an objective (measurable) response to second line salvage chemotherapy[12] . There was no consensus for the treatment of patients with relapsed ALL before the approval of CAR-T cell therapy. The paediatric and young adult population (patients aged up to 25 years) were limited to aggressive chemotherapy regimens, often with limited success.Clinical trial data evaluation
The licensing of CAR-T cells is the result of decades of research. The marketing authorisations were granted based on data from three pivotal clinical trials:ZUMA 1: axicabtagene ciloleucel for diffuse large B-cell lymphoma
This was a phase II multicentre clinical trial conducted in the United States in 2015–2016. Enrolment involved 111 patients with DLBCL and primary mediastinal B-cell lymphoma, or transformed follicular lymphoma with refractory disease, despite undergoing recommended prior therapy. The primary endpoint was objective response, calculated as combined complete and partial response. Axicabtagene ciloleucel was successfully manufactured for 99% (n=110) of the 111 patients, and administered to 91% (n=101) of patients. At 27.1 months, 54% (n=59) of the patients achieved a complete response — also known as complete remission and defined as the disappearance of all signs of cancer in response to treatment[13] . However, this does not always mean the cancer has been cured[14] .ELIANA: tisagenlecleucel for B-cell acute lymphoblastic leukaemia
This was a phase II multicentre global pivotal clinical trial for paediatric and young adults with relapsed/refractory B-cell ALL. Tisagenlecleucel was infused in 75 patients and, at 3 months, there was an overall response of 81%. Overall response describes exhibition of a partial or complete response; although 80% of responders were still in remission at six months, complete response was seen in 60% of patients. Of those patients in remission, 100% had no minimal residual disease detected in their bone marrow. Overall survival was 90% at 6 months and 76% at 12 months[15] .JULIET: tisagenlecleucel for diffuse large B-cell lymphoma
This was a phase II multicentre global pivotal clinical trial for adults with relapsed/refractory DLBCL. A total of 93 patients were followed for at least three months or discontinued earlier. Complete response was seen in 40% of patients and partial response in 12% of patient responses[16] . Partial response — also known as partial remission — is defined as a decrease in the extent of cancer in the body in response to treatment[14] .Training
Implementation of CAR-T cells requires collaboration across the MDT as areas that previously worked independently now work together to ensure safe delivery of CAR-T cells to patients. For instance, pharmacies are required to work closely with stem cell laboratories, which is novel for most pharmacists and requires specialist training on a per-product basis. The scope of training required is broad and is delivered across several disciplines. All staff involved with CAR-T cell therapy (e.g. doctors, nurses, pharmacists and laboratory staff working across haematology, intensive care, neurology and pharmacy) should undertake substantial training in order to understand and be able to deliver the product. In particular, haematology wards, intensive care units, on-call pharmacists and hospital emergency departments are required to have knowledge of CAR-T cells and how to manage complications that may arise as a result of CAR-T cell therapy.Further clinical considerations
Restrictions to medicine prior to chimeric antigen receptor T-cell infusion
Therapeutic doses of steroids should be stopped at least three days before cells are harvested for tisagenlecleucel manufacture and seven days prior to apheresis for axicabtagene ciloleucel manufacture. However, physiological replacement doses are allowed[17] . Pharmacists should be aware of the following:- Steroids and other immunosuppressant drugs should not be used as pre-medication for CAR-T cell therapy or following CAR-T cell infusion. Steroids must be stopped five days or five half-lives, whichever is longer, prior to cell infusion, except if they are required for physiological glucocorticoid replacement therapy where ≤40mg/day hydrocortisone or equivalent is acceptable[18] ;
- Patients may require blood product administration during CAR-T cell treatment as a result of lymphodepletion and/or the nature of their disease. Patients should not have steroids as pre-medication unless approved by a consultant haematologist;
- Granulocyte-macrophage colony-stimulating factor should be avoided owing to its potential to exacerbate toxicities. Short-acting granulocyte-colony stimulating factor (G-CSF) should not be given within 72 hours of cell infusion and long-acting G-CSF should not be given within 10 days of cell infusion[18] .
Toxicities of chimeric antigen receptor T-cell therapy
CAR-T cell therapies are associated with unique toxicities, including:Neurologic toxicities (e.g. headaches and hallucinations);- Hepatic toxicities (e.g. transaminitis [i.e. high levels of liver enzymes called transaminases] and hyperbilirubinemia [i.e. a high level of bilirubin in the blood]);
- Haematologic toxicities (e.g. anaemia and elevated D-dimer — an abnormally high level of cross-linked fibrin degradation products in the body);
- Cardiovascular toxicities (e.g. tachycardia and hypotension);
- Pulmonary toxicities (e.g. tachypnoea and hypoxia)
- Renal toxicities (e.g. acute kidney injury and tumour lysis syndrome [i.e. when tumour cells release their contents into the bloodstream]);
- Gastrointestinal toxicities (e.g. nausea and diarrhoea);
- Musculoskeletal toxicities (e.g. weakness and myalgias)[19] .
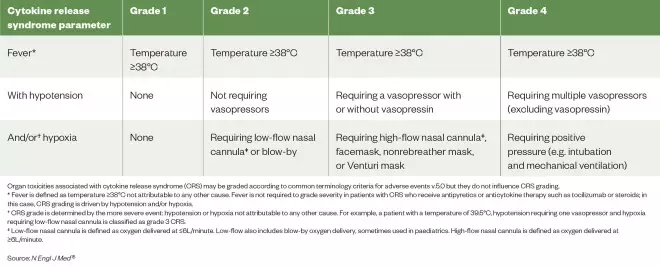
Table 1: American Society for Transplantation and Cellular Therapy cytokine release syndrome consensus grading
Source: N Engl J Med [15]
Organ toxicities associated with cytokine release syndrome (CRS) may be graded according to common terminology criteria for adverse events v.5.0 but they do not influence CRS grading. * Fever is defined as temperature ≥38°C not attributable to any other cause. Fever is not required to grade severity in patients with CRS who receive antipyretics or anticytokine therapy such as tocilizumab or steroids; in this case, CRS grading is driven by hypotension and/or hypoxia. †CRS grade is determined by the more severe event: hypotension or hypoxia not attributable to any other cause. For example, a patient with a temperature of 39.5°C, hypotension requiring one vasopressor and hypoxia requiring low-flow nasal cannula is classified as grade 3 CRS. ‡ Low-flow nasal cannula is defined as oxygen delivered at ≤6L/minute. Low-flow also includes blow-by oxygen delivery, sometimes used in paediatrics. High-flow nasal cannula is defined as oxygen delivered at ≥6L/minute.
Treatment of cytokine release syndrome
CRS is characterised by fever, hypotension and respiratory insufficiency associated with elevated serum cytokines, including interleukin-6 (IL-6), interferon gamma and tumour necrosis factor alpha during the expansion of the infused cells. Symptoms usually occur within 1–14 days following infusion, and are most frequent and more severe in patients with high tumour burden[17] . Patients should be monitored daily for the first ten days following infusion for signs and symptoms of CRS[15],[18] . Supportive care and corticosteroids have been used for the effective treatment of CRS, as well as tocilizumab, which is a recombinant humanised monoclonal antibody directed against IL-6[18] . Tocilizumab’s product licence includes the treatment for the signs and symptoms of CRS of grade 2 and above. It is given as a one-hour, weight dependent (12mg/kg for patients weighing less than 30kg or 8mg/kg for patients weighing at least 30kg) intravenous infusion. If there is no improvement in the signs and symptoms of CRS after the first dose, up to three additional doses may be administered. There should be at least eight hours between consecutive doses as per the requirement in the summary of product characteristics. Doses exceeding 800mg per infusion are not recommended in patients with CRS[22] . Cytopenias and elevated liver transaminases can occur in patients owing to underlying malignancy preceding chemotherapy or CRS. Tocilizumab does not require dose modification for elevated liver enzymes in the context of CRS and, in some cases, tocilizumab is not sufficiently effective. Targeted immunosuppressive agents, anakinra or siltuximab should be considered, but neither is currently licensed for this indication and doses have not yet been established on a consensus basis[23] . These off-label medicines can only be used on the advice of a haematology consultant. Each treatment centre will have their own individual funding and supply arrangements if these medicines are required. Pharmacists must ensure that appropriate treatment is promptly available[10] . It is a prerequisite of both of the CAR-T cell companies that four doses of tocilizumab must be available on the ward for each patient to whom CAR-T cell therapy is administered.Immune effector cell-associated neurotoxicity
ICANS may manifest as delirium (caused by encephalopathy), aphasia (language comprehension impairment), lethargy, difficulty in concentrating, agitation, tremor, seizures and, rarely, cerebral oedema. Headache is very common, although its existence does not necessarily represent neurotoxicity in every case[18] . Impairment of attention, cognitive processes and changes in handwriting are early and common signs of ICANS (see Figure 3)[20] . Encephalopathy is measured using the immune effector cell-associated encephalopathy (ICE) score18 (see Box 1, Table 2 and Table 3). The ICE score is used in conjunction with ASTCT toxicity assessment to determine the ICANS grade[20] .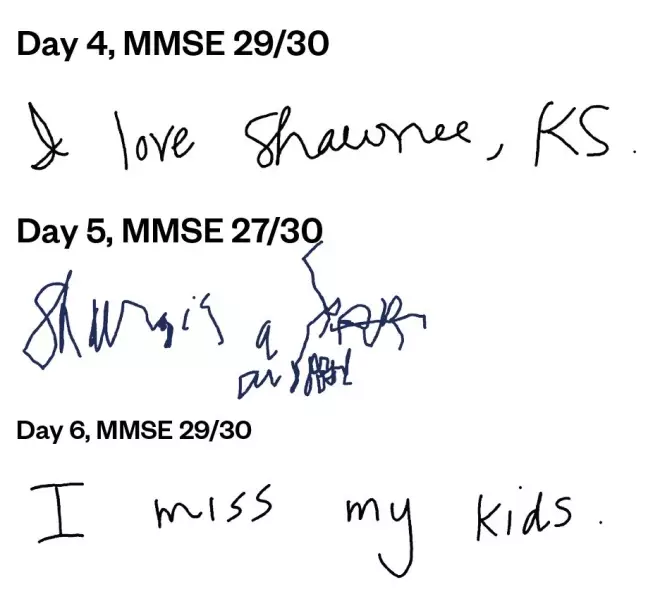
Figure 3: Example of deterioration and improvement in handwriting in a patient with immune effector cell-associated neurotoxicity
MMSE: mini mental state exam
Box 1: Immune-effector cell-associated encephalopathy tool
The patient would be assessed by direct questioning on the following issues:- Orientation: Being able to correctly state the year, month, city and/or hospital — 4 points
- Naming: Their ability to correctly name three objects (e.g. a clock, pen or button) — 3 points
- Following commands: Being able to, for example, show two fingers or close their eyes and stick out their tongue — 1 point
- Writing: Their ability to write a standard sentence (e.g. “I went to the shops to buy milk”— 1 point
- Attention: Count backwards from 100 in units of 10 — 1 point [20]
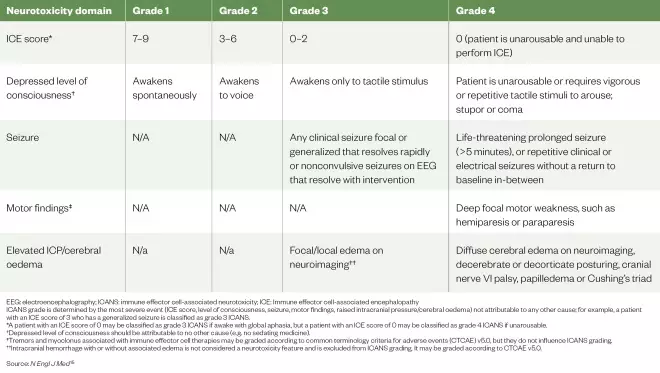
Table 2: The American Society for Transplantation and Cellular Therapy immune effector cell associated neurotoxicity consensus grading for adults
Source: N Engl J Med [15]
EEG: electroencephalography; ICANS: immune effector cell-associated neurotoxicity; ICE: Immune effector cell-associated encephalopathy ICANS grade is determined by the most severe event (ICE score, level of consciousness, seizure, motor findings, raised intracranial pressure/cerebral oedema) not attributable to any other cause; for example, a patient with an ICE score of 3 who has a generalized seizure is classified as grade 3 ICANS. *A patient with an ICE score of 0 may be classified as grade 3 ICANS if awake with global aphasia, but a patient with an ICE score of 0 may be classified as grade 4 ICANS if unarousable. †Depressed level of consciousness should be attributable to no other cause (e.g. no sedating medicine). ‡Tremors and myoclonus associated with immune effector cell therapies may be graded according to common terminology criteria for adverse events (CTCAE) v5.0, but they do not influence ICANS grading.††Intracranial hemorrhage with or without associated edema is not considered a neurotoxicity feature and is excluded from ICANS grading. It may be graded according to CTCAE v5.0.
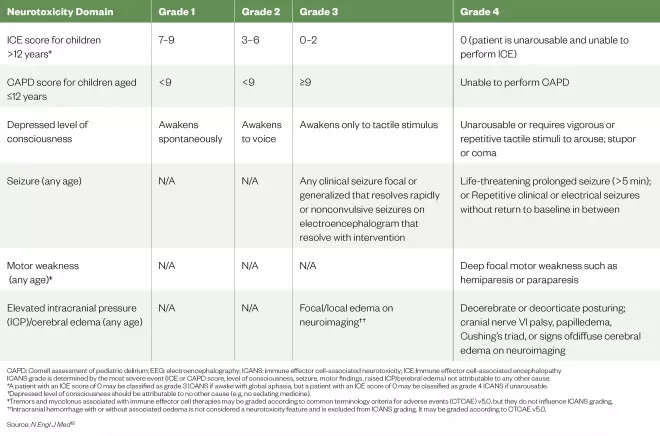
Table 3: The American Society for Transplantation and Cellular Therapy immune effector cell associated neurotoxicity consensus grading for children
Source: N Engl J Med [15]
CAPD: Cornell assessment of pediatric delirium; EEG: electroencephalography; ICANS: immune effector cell-associated neurotoxicity; ICE:Immune effector cell-associated encephalopathy ICANS grade is determined by the most severe event (ICE or CAPD score, level of consciousness, seizure, motor findings, raised ICP/cerebral edema) not attributable to any other cause. *A patient with an ICE score of 0 may be classified as grade 3 ICANS if awake with global aphasia, but a patient with an ICE score of 0 may be classified as grade 4 ICANS if unarousable. †Depressed level of consciousness should be attributable to no other cause (e.g. no sedating medicine). ‡Tremors and myoclonus associated with immune effector cell therapies may be graded according to common terminology criteria for adverse events (CTCAE) v5.0, but they do not influence ICANS grading. ††Intracranial hemorrhage with or without associated oedema is not considered a neurotoxicity feature and is excluded from ICANS grading. It may be graded according to CTCAE v5.0.
Implications for pharmacists
Most side effects of CAR-T cell therapy will occur in the first three to four weeks after infusion, while the patient is being closely monitored, but they may also appear for several months. Early CRS symptoms may be flu-like, which can lead patients or their relatives to go to a community pharmacy or a GP. It is essential, therefore, that all healthcare professionals in primary and secondary care, and at the interfaces, understand this innovative class of medicines and their effects on patients. The evolution of CAR-T cells has been much more closely aligned with processes in place for stem cell transplantation than with those in place for development and handling of small molecule medicines or, indeed, biologics, such as monoclonal antibodies. Therefore, the definition of ATMPs as medicines by Regulation 1394/2007 of the European Parliament and of the Council in 2007 brought with it additional responsibility for pharmacy[25] . As chief pharmacists are routinely the named individual responsible for the safe and secure handling of all medicines within their organisation and are required to be responsible for all medicines used within their healthcare establishment, it is important that they oversee the implementation of CAR-T cell therapy[26] . The role of the pharmacist can be broadly split into three areas: governance, clinical and operational (see Box 2). Medicines governance for CAR-T cells is required at various levels encompassing both national and local requirements. Chief pharmacists have been advised to oversee the local governance arrangements and to ensure that CAR-T cells are of appropriate quality for their intended use[27] .Box 2: Role of the pharmacist in the safe delivery of CAR-T cell therapy
Pharmacists have responsibility for several aspects: Governance- Ensure all appropriate and relevant systems are in place, locally and nationally;
- Collaborate with the clinical multidisciplinary team to ensure the use of medicine is optimised logistically and clinically for patient safety;
- Report to commissioners and ensure that agreed payments are received by the healthcare establishment;
- Manage deviations from the expected pathway — for example, if the patient cannot receive the treatment or the product is out of specification.
- Ensure safe and secure handling of CAR-T cell medicines by providing oversight of operational procedures;
- Create or approve standard operating procedures and associated training
- Ensure patients are eligible for treatment under local and national guidance;
- Verify prescriptions for bridging and conditioning chemotherapy;
- Ensure that appropriate treatment for side effects is promptly available;
- Advise on delivery and doses of supportive medicine;
- Ensure that electronic prescribing systems have correct and accurate prescriptions and delivery of bridging and conditioning chemotherapy.
Pharmacy representation
The Pan-UK Pharmacy Working Group for ATMPs includes representatives from each of the CAR-T cell implementation centres (see Box 3). It has produced ‘pharmacy institutional readiness’ checklists to aid consistency in the implementation of CAR-T across the UK[10] . The Group has also established a clinical pharmacy subgroup that is further defining the clinical pharmacist’s role in this area and producing a standardised toxicity management tool based on clinical experience to date. The group also aims to use horizon scanning data to enable proactive collaboration and to facilitate the implementation of new ATMPs as they become available.Box 3: NHS-commissioned chimeric antigen receptor T-cell therapy centres
Adults (aged over 18 years):- The Christie NHS Foundation Trust;
- University Hospitals Birmingham (Queen Elizabeth Hospital);
- University College London Hospitals NHS Foundation Trust*;
- King’s College Hospital NHS Foundation Trust;
- University Hospitals Bristol NHS Foundation Trust;
- Newcastle upon Tyne Hospitals NHS Foundation Trust (Freeman Hospital);
- Manchester University NHS Foundation Trust (Manchester Royal Infirmary);
- Public Health Wales NHS Trust (University Hospitals of Wales).
- Newcastle upon Tyne Hospitals NHS Foundation Trust (Great North Children’s Hospital);
- Manchester University NHS Foundation Trust (Royal Manchester Children’s Hospital).
Box 4: Case study of a patient receiving chimeric antigen receptor T-cell therapy
Alex* is a male aged 18 years with primary mediastinal B-cell lymphoma, which is a type of diffuse large B-cell lymphoma. He was deemed to be eligible for chimeric antigen receptor T-cell (CAR-T cell) treatment under NHS England criteria by the national CAR-T multidisciplinary team as his local hospital did not offer CAR-T cell therapy. The nearest available centre was approximately three hours’ travel from his home. Treatment Alex was treated with dose-adjusted rituximab, etoposide, prednisolone, vincristine, cyclophosphamide and doxorubicin, given for six cycles between July 2017 and November 2017, when he was aged 16 years. Partial remission repeat positron emission tomography (PET)** showed further progression in July 2018. His treatment schedule continued as follows until April 2019.- July 2018: Ifosfamide, gemcitabine and vinorelbine given for two cycles;
- September 2018: Given radiotherapy;
- January 2019: Progressive disease;
- February 2019: Referred for CAR-T cells harvesting. Patient quite well at the time and had ECOG*** score of 0;
- Late February 2019: Apheresis;
- March 2019: Conditioning chemotherapy with fludarabine and cyclophosphamide given in order to prepare the body for CAR-T cells, as specified in the summary of product characteristics;
- April 2019: Axicabtagene ciloleucel (CAR-T cell) infusion.
- Co-trimoxazole 960mg taken on Mondays, Wednesdays and Fridays;
- Aciclovir 200mg taken three times per day;
- Levetiracetam 1000mg twice-daily (stopped 14 days after commencing on neurology advice).
- Partial response on one-month PET scan;
- Three-month PET progressive disease;
- Subsequently received chemo-immunotherapy, but disease progressed;
- Unfortunately, Alex has now undergone an allogeneic (donor) stem cell transplant owing to insufficient response to CAR-T treatment.
References
[1] eMC. Kymriah cells dispersion for infusion. 2020. Available at: https://www.medicines.org.uk/emc/product/9456 (accessed May 2020)
[2] eMC. Yescarta (axicabtagene ciloleucel) dispersion for infusion. 2020. Available at: https://www.medicines.org.uk/emc/product/9439 (accessed May 2020)
[3] NHS England. NHS England strikes deal for ground breaking cancer treatment in a new European first. 2018. Available at: https://www.england.nhs.uk/2018/10/nhs-england-strikes-deal-for-ground-breaking-cancer-treatment-in-a-new-european-first (accessed May 2020)
[4] National Institute for Health and Care Excellence. Axicabtagene ciloleucel ciloleucel for treating diffuse large B-cell lymphoma and primary mediastinal large B-cell lymphoma after 2 or more systemic therapies. 2019. Available at: https://www.nice.org.uk/guidance/ta559/resources/axicabtagene ciloleucel-ciloleucel-for-treating-diffuse-large-bcell-lymphoma-and-primary-mediastinal-large-bcell-lymphoma-after-2-or-more-systemic-therapies-pdf-82607030270917 (accessed May 2020)
[5] National Institute for Health and Care Excellence. Tisagenlecleucel for treating relapsed or refractory B-cell acute lymphoblastic leukaemia in people aged up to 25 years. 2018. Available at: https://www.nice.org.uk/guidance/ta554/resources/tisagenlecleucel-for-treating-relapsed-or-refractory-bcell-acute-lymphoblastic-leukaemia-in-people-aged-up-to-25-years-pdf-82607021872837 (accessed May 2020)
[6] NHS England. CAR-T Therapy. 2018. Available at: https://www.england.nhs.uk/cancer/cdf/car-t-therapy (accessed May 2020)
[7] Wang X & Riviere I. Clinical manufacturing of CAR-T cells: foundation of a promising therapy. Mol Ther Oncolytics 2016;3:106015. doi: 10.1038/mto.2016.15
[8] Restifo NP, Dudley ME & Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T-cell response. Nat Rev Immmunol 2012;12(4):269–281. doi: 10.1038/nri3191
[9] NHS Specialist Pharmacy Service. Regulatory Requirements for export of ATMP Starting materials: Pan UK Pharmacy Working Group on ATMPs. 2019. Available at: https://www.sps.nhs.uk/articles/regulatory-requirements-for-export-of-atmp-starting-materials-pan-uk-pharmacy-working-group-on-atmps (accessed May 2020)
[10] NHS Specialist Pharmacy Service. Pharmacy Institutional Readiness for Marketed CAR-T therapy: Guidance if Chief Pharmacists. 2018. Available at: https://www.sps.nhs.uk/articles/pharmacy-institutional-readiness-for-marketed-car-t-therapy-guidance-for-chief-pharmacists (accessed May 2020)
[11] Hay K & Turtle C. Chimeric antigen receptor (CAR) T cells: lessons learned from targeting of CD19 in B cell malignancies. Drugs 2017;77(3):237–245. doi: 10.1007/s40265-017-0690-8
[12] Gisselbrecht C, Glass B, Mounier N et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010;28(27):4184–4190 doi: 10.1200/JCO.2010.28.1618
[13] Neelapu SS, Locke FL, Bartlett NL et al. Axicabtagene ciloleucel ciloleucel CAR-T cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;37(26)7:2531–2544. doi: 10.1056/NEJMoa1707447
[14] National Cancer Institute. NCI dictionary of cancer terms. 2009. Available at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/partial-response (accessed May 2020)
[15] Maude SL, Laetsch TW Buechner J et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866
[16] Schuster SJ, Bishop MR, Tam CS et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980
[17] NHS Specialist Pharmacy Service. Medication-restrictions for patients having CAR-T cell therapy. 2020. Available at: https://www.sps.nhs.uk/articles/medication-restrictions-for-patients-having-car-t-cell-therapy (accessed May 2020)
[18] Lee DW, Gardner R, Porter DL et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729
[19] Brudno JN & Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016;127(26):3321–3330. doi: 10.1182/blood-2016-04-703751
[20] Lee DW, Santomasso BD, Locke FLet al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758
[21] Foundation for the Accreditation of Cellular Therapy & Joint Accreditation Committee ISCT-EMBT. FACT-JACIE international standards for hematopoietic cellular therapy. 2018. Available at: https://www.ebmt.org/sites/default/files/2018-06/FACT-JACIE%207th%20Edition%20Standards.pdf (accessed May 2020).
[22] eMC. RoActemra 20mg/mL concentrate for solution for infusion. 2019. Available at: https://www.medicines.org.uk/emc/product/6673/smpc (accessed May 2020)
[23] Service. Evidence for use of siltuximab or anakinra as second line therapies (after failure of tocilizumab) for cytokine release syndrome following use of chimeric antigen receptor T-cell (CAR-T) therapy. 2020. Available at: https://www.sps.nhs.uk/articles/evidence-for-use-of-siltuximab-or-anakinra-as-second-line-therapies-after-failure-of-tocilizumab-for-cytokine-release-syndrome-following-use-of-chimeric-antigen-receptor-t-cell-car-t-therapy (accessed May 2020)
[24] Santomasso B, Bachier C, Westin J et al. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity and financial burden. Am Soc Clin Oncol Educ Book 2019;39:433–444. doi: 10.1200/EDBK_238691
[25] EUR-Lex. European parliament and council regulation (EC) no 1394/2007 on advanced therapy medicinal products. 2007. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2007.324.01.0121.01.ENG&toc=OJ:L:2007:324:TOC (accessed May 2020)
[26] Royal Pharmaceutical Society. Professional guidance on the safe and secure handling of medicines. 2018. Available at: https://www.rpharms.com/recognition/setting-professional-standards/safe-and-secure-handling-of-medicines/professional-guidance-on-the-safe-and-secure-handling-of-medicines (accessed May 2020)
[27] NHS Specialist Pharmacy Service. The role of pharmacy in the successful delivery of advanced therapy medicinal products. 2019. https://www.sps.nhs.uk/articles/atmps-the-role-of-pharmacy-in-the-successful-delivery-of-advanced-therapy-medicinal-products-atmps-information-for-chief-pharmacists (accessed May 2020)
[28] NHS Specialist Pharmacy Service. Out of specification advanced therapy medicinal products — guidance for healthcare organisations. 2020. Available at: https://www.sps.nhs.uk/articles/out-of-specification-advanced-therapy-medicinal-products-guidance-for-healthcare-organisations (accessed May 2020)
[29] NHS. PET scan. 2018. Available at: https://www.nhs.uk/conditions/PET-scan (accessed May 2020)
[30] ECOG ACRIN Cancer Research Group. ECOG performance status. 2015. Available at: https://ecog-acrin.org/resources/ecog-performance-status (accessed May 2020)
[31] Restifo NP, Dudley ME & Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 2012;12(4):269281. doi: 10.1038/nri3191