JL / Shutterstock.com
Acne is a common skin condition that affects 90% of people at some point during their teenage years[1]
. Although predominately an adolescent condition, acne can persist into adult life. Estimates of the prevalence in those aged 40 years and over have ranged from 26% in females and 12% in males, to more than 40% in both sexes[2],[3]
. Research suggests that acne can be associated with significant psychological morbidity, impair the individual’s quality of life and can lead to suicide ideation[4],[5],[6]
.
Pathophysiology
Acne is a disease of the pilosebaceous follicles (PSF) that are present over the surface of the skin, except for the palms of the hands and soles of the feet. There is a much higher concentration of PSF in the T-zone of the face (i.e. the forehead and nose), the chest and the back.
Although the precise cause of acne remains unclear, at least four factors are implicated in the pathophysiology:
- The release of inflammatory agents in the skin;
- Proliferation of the anaerobic organism Cutibacterium acnes (formerly known as Propionibacterium acnes), which is present within functionally blocked follicles;
- An increased production of altered sebum;
- Abnormal keratinocyte proliferation and differentiation (ductal hypercornification or comedogenesis).
An inflammatory response is also believed to precede abnormal keratinocyte proliferation[7]
. C.
acnes can activate toll-like receptors (TLRs) that are present on the surface of keratinocytes and monocytes. These receptors form part of the innate immune system, and the stimulation of TLRs on monocytes and keratinocytes release the pro-inflammatory cytokines interleukin (IL)-8 and IL-12[8]
.
In addition, C. acnes has several other effects, including the production of lipase enzymes that act as chemoattractant for neutrophils[9]
and the release of tumour necrosis factor alpha and various proteases and hyaluronidases that contribute towards tissue injury[10],[11]
(see Figure 1 and Figure 2).
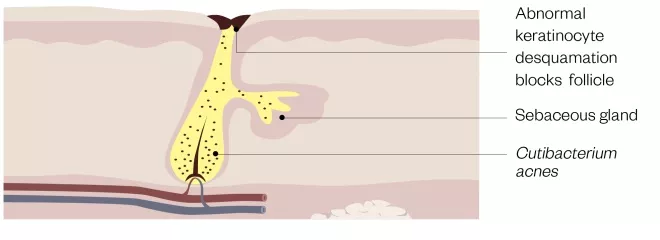
Figure 1: Structure of a blocked pilosebaceous follicle
The androgen-mediated increased output of altered sebum serves as a substrate for Cutibacterium acnes, which degrades sebum triglycerides, releasing irritant fatty acids that may induce comedogenesis. Epithelial keratinocytes lining the pilosebaceous follicle undergo abnormal desquamation and block the follicular opening. Increased production of altered sebum allows C. acnes to flourish in a blocked follicle
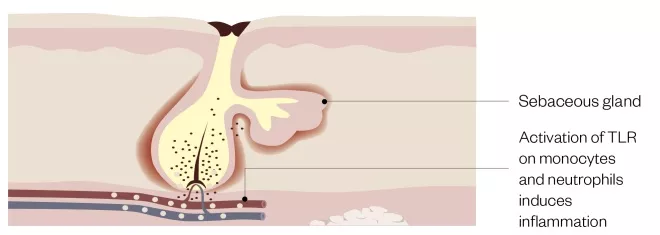
Figure 2: Actions of Cutibacterium acnes
Cutibacterium acnes within the blocked follicle activates toll-like receptors (TLRs) on monocytes and keratinocytes inducing release of pro-inflammatory cytokines. Additional agents produced by C. acnes contribute towards tissue damage as well as initiation of comedogenesis
The onset of adolescence is characterised by an increase in the production of sex hormones (mainly androgens) that cause sebaceous gland hyperplasia with a corresponding increase in the output of sebum. This gives the skin a greasy appearance and, while this is the first clinical symptom of acne, it is not a prerequisite. It is believed that alterations in the composition of sebum, rather than the quantity, is associated with acne.
Changes in sebum composition include increased levels of squalene, alterations in the ratio of fatty acids and a reduction in the levels of linoleic acid[12]
. Sebum serves as a substrate for C. acnes, which produces a lipase enzyme that hydrolyses sebum triglycerides into glycerol and free fatty acids[13]
. Two fatty acids — palmitic acid and oleic acid — appear to be involved in the production of pro-inflammatory cytokines, which can contribute to inflammation in acne, as well as ductal hypercornification[14],[15]
.
The final factor is abnormal desquamation (shedding) of the epithelial keratinocytes lining the PSF. The main driver for comedogenesis remains unclear but in
vitro work with isolated sebaceous glands has shown that peptidoglycan, a component of the C. acnes cell wall, activates keratinocyte TLR-2, stimulating the release of interleukin-1 (IL-1), which leads to comedone formation[16]
. Indeed, the addition of IL-1 to isolated PSFs leads to comedone formation[17]
.
Types of acne
The main inflammatory lesions (see Figure 3) are:
- Papules — erythematous lesions, less than 5mm in diameter;
- Pustules — white/yellow-fluid-filled lesions, less than 5mm in diameter;
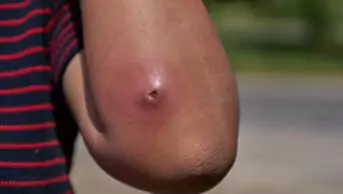
- Nodules — painful, solid lesions deep within the skin, larger than 5mm in diameter, with varying degrees of erythema. Can lead to scarring;
- Cysts — similar to nodules but filled with pus. Can lead to scarring.
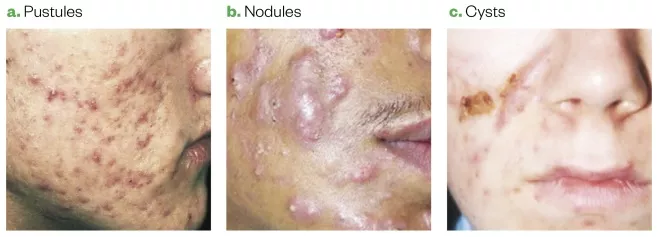
Figure 3: Types of acne
Source: Source: Primary Care Dermatology Society
Comedogenesis leads to the blockage of the PSF and the earliest acne lesion, a ‘microcomedone’, from which all other acne lesions subsequently develop. If this blockage occurs close to the surface of the skin, the resultant keratinocyte plug causes distension of the follicular orifice and melanin present within the desquamated cells reacts with the atmosphere and turns black. This produces open comedone (known as a ‘blackhead’; see Figure 4), whereas if the blockage occurs within the lumen of the PSF, then a closed comedone (or ‘whitehead’) is formed. Closed comedones can remain stable or progress to become inflammatory lesions.
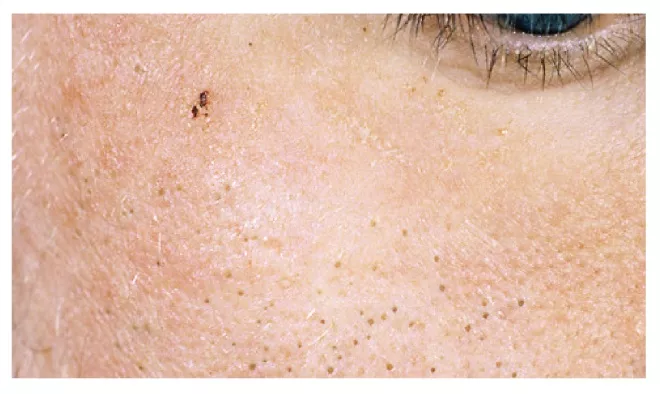
Figure 4: Open comedones
Source: St Bartholomew’s Hospital / Science Photo Library
Causes of acne
Genetics
There is evidence for a link between genes and acne. By using genetic modelling based on acne severity scores, one study showed that 81% of the variance of acne could be attributed to genetic factors[18]
. Furthermore, a case-controlled study in patients in China found that the risk of acne occurring in a relative of a patient with acne was significantly greater than in unaffected relatives[19]
. A more recent study identified further genomic loci at which genetic variation is associated with acne susceptibility[20]
.
Diet
Patients often perceive that diet has an impact on acne, with one study reporting that 92% of patients felt that diet affected their condition[21]
. Although the evidence base is controversial, foods with a high glycaemic index (e.g. white bread, cakes and biscuits), as well as milk and dairy products, are thought to aggravate acne[22],[23],[24]
. It has been suggested that consuming high glycaemic index foods results in an insulin-triggered rise in free insulin-like growth factor-1 (IFG-1), which elevates androgens, sebum synthesis and, ultimately, the development of acne[25]
. Indeed, it has been found that patients with acne consume more carbohydrate[26]
and that lowering the glycaemic index of the diet reduces levels of IGF-1[27]
. Furthermore, when patients with Laron syndrome, who lack IGF-1, were treated with IGF-1, they developed acne[28]
.
Drug-induced acne
A wide range of drugs have been documented as causing an acne-like eruption, including oral corticosteroids, anabolic steroids, progestogens, lithium and anti-epileptics[29]
. Clinically, drug-induced acne is defined by a sudden onset of a monomorphic eruption of inflammatory papules and pustules that are pruritic and follicular, with an absence of comedones[30]
.
Hormones
Polycystic ovary syndrome (PCOS) is a common endocrine disorder of unknown aetiology[31]
. PCOS often first presents during adolescence alongside hyperandrogenism symptoms, such as acne, and hirsutism and/or anovulation. Acne occurs in 10–34% of women with PCOS[32]
.
Lifestyle factors
Several lifestyle factors thought to be associated with acne include:
- Smoking — there is conflicting evidence about the link between smoking and acne; one study found that older women who are smokers have a greater incidence of comedonal acne[33]
, whereas another study found no association[34]
; - Cosmetics — it is considered that cosmetics can worsen acne severity by effectively blocking the PSFs[35]
. It is generally advised that patients use make-up products that are labelled ‘non-comedogenic’; - Stress —
evidence suggests that acne can worsen during periods of stress[36]
. One study identified that sebocytes have receptors for corticotrophin-releasing hormone and other neuroendocrine factors, which may explain the link[37]
.
Case studies
Case study 1: mild acne
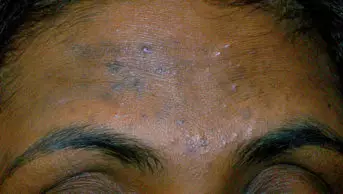
Jessica* is a 15-year-old girl who attends the pharmacy one day after school. She spends time looking at the shelves of facial washes. You approach her and ask if she needs any assistance. She asks for something to treat her spots and you offer to take her into the consultation room. On examination, you notice that she has several open and closed comedones on her face with a small number of inflammatory lesions. When asked, she says that she does not have acne on her chest or back.
Jessica asks you to recommend a suitable facial wash to help get rid of her spots.
How should you respond?
Assessment and diagnosis
Your examination has revealed that the patient has mild, predominantly comedonal acne.
Treatment options
Guidelines state that a topical retinoid, benzoyl peroxide (BPO) or azelaic acid show the best efficacy in mild-to-moderate comedonal acne[38]
. Therefore, a BPO formulation would be an appropriate initial over-the-counter treatment and is available as a 5% gel or a 10% wash. The drug acts via oxidation and the formation of free radicals producing comedolytic, antibacterial and anti-inflammatory effects[39]
.
Advice and recommendations
The patient should be warned about common side effects, such as irritation and bleaching of fabrics[40]
. Recommend that they use an adapalene-BPO gel and suggest that it is applied roughly 15 minutes after washing with soap and warm water, and then removed a few hours later to minimise irritation[41]
.
If the patient prefers to use a wash, the product should be applied to the skin and left in contact for a few minutes before rinsing off. The dryness or skin irritation from BPO can be managed using emollients, in particular, products labelled as ‘oil-free’ or ‘non-comedogenic’, which often contain either dimethicone or cyclomethicone[42]
.
Although the patient wants to use a facial cleanser, the value of these agents in acne remains unclear, with one review concluding that it was difficult to make any firm recommendations given the lack of well-performed studies[43]
. However, a recent study using a 5% BPO gel combined with a cleanser and sun protection moisturiser resulted in high levels of patient satisfaction and treatment efficacy[44]
.
The patient should be advised to apply BPO to the whole of the affected area rather than individual lesions — histological evidence shows that ‘normal’ looking skin has features of microcomedones[45]
.
Finally, advise the patient to continue with the treatment for at least six weeks (a large randomised trial found that most of the treatment benefits occurred within this time frame)[46]
.
Potential outcome of the advice
If there is no response to the treatment after six weeks or if the acne worsens, the patient should be referred to their GP.
Case study 2: moderate acne
Jonathan* is a 30-year-old local estate agent who works near your pharmacy. He has had with acne since adolescence but has never really bothered treating his condition. However, he has recently started a new relationship and is feeling embarrassed about his acne and asks you for an effective cream.
How should you respond?
Assessment and diagnosis
During a private consultation, the patient removes his shirt and, in addition to many inflamed lesions on his face, you notice a large number of papules on his back and chest, as well as the presence of some scarring on his face and chest.
Treatment options
Owing to the difficulty in applying topical treatments over a widespread area, referral to a GP is required. In addition, the acne has already caused some scarring, which is common, for instance, one study of nearly 1,000 patients found some level of facial scarring in 87% of patients[47]
.
The most appropriate treatment for this patient would be the use of an oral antibiotic – either doxycycline or lymecycline (in preference to minocycline or tetracycline) – combined with a topical retinoid[38]
. Benzoyl peroxide (BPO) can be added to the regime to help reduce the likelihood of resistance developing to the oral antibiotic[48]
.
Typically, doxycycline 50–100mg daily and lymecycline 300mg daily are prescribed. Oral antibiotics can have anti-inflammatory effects, including inhibition of neutrophil chemotaxis and inhibition of pro-inflammatory cytokines[49]
. Topical retinoids, such as adapalene, tretinoin and isotretinoin, bind to membrane-bound retinoic acid receptors and this leads to normalisation of keratinocyte differentiation and cohesion, comedolysis and inhibition of comedone formation[50]
.
Advice and recommendations
Common adverse effects experienced by those taking oral antibiotics include vaginal candidiasis (in women), gastrointestinal upset and phototoxicity. Therefore, patients should be advised to take doxycycline with food to avoid UV exposure[51]
.
Topical retinoids are also associated with adverse effects, including erythema, dryness, peeling and/or burning within the first two weeks of therapy[52]
. In an effort to minimise these effects, advise the patient to apply a pea-sized amount of the retinoid to the entire affected area, every other day for the first 2–4 weeks of treatment. Furthermore, the retinoid can be left in contact with the skin for 30–60 minutes before washing off. Any further irritancy can be managed by applying a non-comedogenic emollient[53]
.
Potential outcome of the advice
Using the combination regime, the patient should expect to see some response after 6–8 weeks and, ideally, oral agents should not be continued for longer than 12 weeks[40]
. If treatment with oral agents is ineffective, the patient should be referred for specialist assessment.
Case study 3: polycystic ovary syndrome-induced acne
Anna* is an overweight 17-year-old student who has recently been diagnosed with polycystic ovary syndrome (PCOS). She comes to the pharmacy with a new prescription for co-cyprindiol. You know from your records that Anna has previously had acne; she was prescribed doxycycline but it was ineffective. Anna mentions that she did not get much time to discuss her new diagnosis with the GP or how to take her new medicine, and asks you for advice.
How should you respond?
Advice and recommendations
There are no treatments specifically licensed for PCOS. Co-cyprindiol is given to manage the patient’s acne and is a suitable first-line treatment when someone is not planning to conceive. Co-cyprindiol serves a dual function, being an effective contraceptive (owing to ethinylestradiol), which helps to normalise menstrual irregularities, and an anti-androgenic agent, cyproterone acetate. This latter component competes with dihydrotestosterone for androgen receptors and, therefore, reduces testosterone production, which will improve acne and any hirsutism[31]
. Alternatives to co-cyprindiol include combined oral contraceptives, which are also effective in treating acne[54]
.
The patient should be advised to start taking their co-cyprindiol on the first day of their next period for 21 days and then to have a 7-day pill-free interval. Nausea and abdominal pain are common side effects and, therefore, advise the patient that if they experience any vomiting or diarrhoea, it can reduce the effectiveness of the contraceptive and additional protection should be used for at least seven days.
The drug needs to be continued for at least three months before any likely benefit can be seen. There is a risk of venous thromboembolism with co-cyprindiol; however, recent advice suggests that while the risk is 1.5–2.0 times greater with co-cyprindiol than contraceptives that contain levonorgestrel, the benefits of treatment outweigh the risks[55]
.
Finally, it is helpful to advise the patient that lifestyle measures, such as a healthy diet, regular exercise and achieving a healthy weight, can improve symptoms of hyperandrogenism[56]
.
*All cases are fictional.
References
[1] Dawson AL & Dellavalle R. Acne vulgaris. BMJ 2013;f2634. doi: 10.1136/bmj.f2634
[2] Collier CN, Harper JC, Cafardi JA et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol 2008;58(1):56–59. doi: 10.1016/j.jaad.2007.06.045
[3] Semedo D, Ladeiro F, Ruivo M et al. Adult acne: prevalence and portrayal in primary healthcare patients, in the greater Porto area, Portugal. Acta Med Port 2016;29(9):507–513. doi: 10.20344/amp.6626
[4] Niemeier V, Kupfer J & Gieler U. Acne vulgaris — Psychosomatische aspekte. J Ger Soc Dermatology 2006;4(12):1027–1036. doi: 10.1111/j.1610-0387.2006.06110_suppx.x
[5] Fried RG & Wechsler A. Psychological problems in the acne patient. Dermatol Ther 2006;19:237–40. doi: 10.1111/j.1529-8019.2006.00079.x
[6] Halvorsen JA, Stern RS, Dalgard F et al. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population-based study. J Invest Dermatol 2011;131(2):363–370. doi: 10.1038/jid.2010.264
[7] Jeremy AH, Holland DB, Roberts SG et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol 2003;121(1):20–27. doi: 10.1046/j.1523-1747.2003.12321.x
[8] Beylot C, Auffret N, Poli F et al. Propionibacterium acnes: an update on its role in the pathogenesis of acne. J Eur Acad Dermatology Venereol 2014;28(3):271–278. doi: 10.1111/jdv.12224
[9] Lee WL, Shalita AR, Suntharalingam K & Fikrig SM. Neutrophil chemotaxis by Propionibacterium acnes lipase and its inhibition. Infect Immun 1982;35(1):71–78. PMID: 7054130
[10] Graham GM, Farrar MD, Cruse-Sawyer JE et al. pro-inflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br J Dermatol 2004;150(3):421–428. doi: 10.1046/j.1365-2133.2004.05762.x
[11] Dessinioti C & Katsambas AD. The role of Propionibacterium acnes in acne pathogenesis: facts and controversies. Clin Dermatol 2010;28(1):2–7. doi: 10.1016/j.clindermatol.2009.03.012
[12] Li X, He C, Chen Z et al. A review of the role of sebum in the mechanism of acne pathogenesis. J Cosmet Dermatol 2017;16(2):168–173. doi: 10.1111/jocd.12345
[13] Holland C, Mak TN, Zimny-Arndt U et al. Proteomic identification of secreted proteins of Propionibacterium acnes. BMC Microbiol 2010;10:230. doi: 10.1186/1471-2180-10-230
[14] Zhou BR, Zhang JA, Zhang Q et al. Palmitic acid induces production of pro-inflammatory cytokines interleukin-6, interleukin-1β, and tumor necrosis factor-α via a NF-κB-dependent mechanism in HaCaT keratinocytes. Mediators Inflamm 2013;2013:530429. doi: 10.1155/2013/530429
[15] Katsuta Y, Lida T, Hasegawa K et al. Function of oleic acid on epidermal barrier and calcium influx into keratinocytes is associated with N-methyl d-aspartate-type glutamate receptors. Br J Dermatol 2009;160(1):69–74. doi: 10.1111/j.1365-2133.2008.08860.x
[16] Selway JL, Kurczab T, Kealey T & Langlands K. Toll-like receptor 2 activation and comedogenesis: Implications for the pathogenesis of acne. BMC Dermatol 2013;13(1):10. doi: 10.1186/1471-5945-13-10
[17] Guy R, Green MR & Kealey T. Modeling acne in vitro. J Invest Dermatol. 1996;106(1):176–182. PMID: 8592071
[18] Bataille V, Snieder H, MacGregor AJ et al. The influence of genetics and environmental factors in the pathogenesis of acne: a twin study of acne in women.J Invest Dermatol 2002;119(6):1317–1322. doi: 10.1046/j.1523-1747.2002.19621.x
[19] Xu SX, Wang HL, Fan X et al. The familial risk of acne vulgaris in Chinese Hans — A case-control study. J Eur Acad Dermatology Venereol 2007;21(5):602–605. doi: 10.1111/j.1468-3083.2006.02022.x
[20] Petridis C, Navarini AA, Dand Net al. Genome-wide meta-analysis implicates mediators of hair follicle development and morphogenesis in risk for severe acne. Nature Communications 2018;9(5075). doi: 10.1038/s41467-018-07459-5
[21] Nguyen Q-G, Markus R & Katta R. Diet and acne: an exploratory survey study of patient beliefs. Dermatol Pract Concept 2016;6(2):21–27. doi: 10.5826/dpc.0602a05
[22] Mahmood SN & Bowe W. Diet and acne update: carbohydrates emerge as the main culprit. J Drugs Dermatol 2014;13(4):428–435. PMID: 24719062
[23] Dai R, Hua W, Chen W et al. The effect of milk consumption on acne: a meta-analysis of observational studies. J Eur Acad Dermatology Venereol 2018;32(12):2244–2253. doi: 10.1111/jdv.15204
[24] Fiedler F, Stangl GI, Fiedler E & Taube KM. Acne and nutrition: a systematic review. Acta Derm Venereol 2017;97(1):7–9. doi: 10.2340/00015555-2450
[25] Cordain L, Eades MR & Eades MD. Hyperinsulinemic diseases of civilization: more than just Syndrome X. Comp Biochem Physiol — A Mol Integr Physiol 2003;136(1):95–112. PMID: 14527633
[26] Burris J, Rietkerk W, Shikany JM & Woolf K. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet 2017;117(9):1375–1383. doi: 10.1016/j.jand.2017.03.024
[27] Burris J, Shikany JM, Rietkerk W & Woolf K. A low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet 2018;118(10):1874–1885. doi: 10.1016/j.jand.2018.02.009
[28] Ben-Amitai D & Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatology Venereol 2011;25(8):950–954. doi: 10.1111/j.1468-3083.2010.03896.x
[29] Dy-Thanh A, Kluger N, Bensalleh H & Guillot B. Drug-induced acneiform eruption. Am J Clin Dermatol 2011;12(4):233–245. doi: 10.2165/11588900-000000000-00000
[30] Pontello Junior R & Kondo RN. Drug-induced acne and rose pearl: similarities. An Bras Dermatol 2013;88(6):1039–1040. doi: 10.1590/abd1806-4841.20132586
[31] Buzney E, Sheu J, Buzney C & Reynolds RV. Polycystic ovary syndrome: a review for dermatologists. Part II. Treatment. J Am Acad Dermatol 2014;71(5):859.e1-859.e15. doi: 10.1016/j.jaad.2014.05.009
[32] Chuan SS & Chang RJ. Polycystic ovary syndrome and acne. Skin Therapy Lett 2010;15(10):1–4. PMID: 21076799
[33] Capitanio B, Sinagra JL, Ottaviani M et al. Acne and smoking. Dermatoendocrinol 2009;1(3):129–135. PMID: 20436880
[34] Firooz A, Sarhangnejad R, Davoudi SM & Nassiri-Kashani M. Acne and smoking: Is there a relationship?BMC Dermatol 2005;5:11–13. doi: 10.1186/1471-5945-5-2
[35] Perera MPN, Peiris WMDM, Pathmanathan D et al. Relationship between acne vulgaris and cosmetic usage in Sri Lankan urban adolescent females. J Cosmet Dermatol 2018;17(3):431–436. doi: 10.1111/jocd.12431
[36] Yosipovitch G, Tang M, Dawn AG et al. Study of psychological stress, sebum production and acne vulgaris in adolescents. Acta Derm Venereol 2007;87(2):135–139. doi: 10.2340/00015555-0231
[37] Zouboulis CC & Bohm M. Neuroendocrine regulation of sebocytes — a pathogenetic link between stress and acne. Exp Dermatol 2004;13(s4):31–35. doi: 10.1111/j.1600-0625.2004.00254.x
[38] Bettoli V, Mokos ZB, Degitz Ket al. European evidence-based (S3) guideline for the treatment of acne – update 2016 – short version. J Eur Acad Dermatol Venereol 2016;30:1261–1268. doi: 10.1111/jdv.13776
[39] Masterson KN. Acne basics: pathophysiology, assessment, and standard treatment options. J Dermatology Nurses’ Assoc 2018;10(2008):S2–10. doi: 10.1097/JDN.0000000000000361
[40] Zaenglein AL, Pathy AL, Schlosser BJ et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol 2016;74(5):945–973e33. doi: 10.1016/j.jaad.2015.12.037
[41] Patient UK. Benzoyl peroxide for acne. 2018. Available at: https://patient.info/medicine/benzoyl-peroxide-for-acne-acnecide-brevoxyl (accessed June 2019)
[42] Chularojanamontri L, Tuchinda P, Kulthanan K & Pongparit K. Moisturizers for acne: what are their constituents? J Clin Aesthet Dermatol 2014;7(5):36–44. PMID: 24847408
[43] Stringer T, Nagler A, Orlow SJ & Oza VS. Clinical evidence for washing and cleansers in acne vulgaris: a systematic review. J Dermatolog Treat 2018;29(7):688–693. doi: 10.1080/09546634.2018.1442552
[44] Kim MR & Kerrouche N. Combination of benzoyl peroxide 5% gel with liquid cleanser and moisturizer SPF 30 in acne treatment results in high levels of subject satisfaction, good adherence and favorable tolerability. J Dermatolog Treat 2018;29(1):49–54. doi: 10.1080/09546634.2017.1342758
[45] Saurat JH. Strategic targets in acne: the comedone switch in question. Dermatology 2015;231(2):105–111. doi: 10.1159/000382031
[46] Ozolins M, Eady EA, Avery AJ et al. Comparison of five antimicrobial regimens for treatment of mild to moderate inflammatory facial acne vulgaris in the community: randomised controlled trial. Lancet 2004;364(9452):2188–2195. doi: 10.1016/S0140-6736(04)17591-0
[47] Tan JK, Tang J, Fung K et al. Development and validation of a scale for acne scar severity (SCAR-S) of the face and trunk. J Cutan Med Surg 2010;14(4):156–160. PMID: 20642983
[48] Bienenfeld A, Nagler AR & Orlow SJ. Oral antibacterial therapy for acne vulgaris: an evidence-based review. Am J Clin Dermatol 2017;18(4):469–490. doi: 10.1007/s40257-017-0267-z
[49] Sapadin AN & Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol 2006;54(2):258–265. doi: 10.1016/j.jaad.2005.10.004
[50] Das S & Reynolds RV. Recent advances in acne pathogenesis: implications for therapy. Am J Clin Dermatol 2014;15(6):479–488. doi: 10.1007/s40257-014-0099-z
[51] Oudenhoven MD, Kinney MA, McShane DB et al. Adverse effects of acne medications: recognition and management. Am J Clin Dermatol 2015;16(4):231–242. doi: 10.1007/s40257-015-0127-7
[52] Culp L, Moradi Tuchayi S, Alinia H & Feldman SR. Tolerability of topical retinoids: are there clinically meaningful differences among topical retinoids? J Cutan Med Surg 2015;19(6):530–538. doi: 10.1177/1203475415591117
[53] Thiboutot DM, Abanmi A, Alexis AF et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol 2018;78(2):S1–S23.e1. doi: 10.1016/j.jaad.2017.09.078
[54] Arrington EA, Patel NS, Gerancher K & Feldman SR. Combined oral contraceptives for acne: a practical guide. Cutis 2012;90(2):83–90. PMID: 22988652
[55] Medicines and Healthcare products Regulatory Agency. Cyproterone acetate with ethinylestradiol (co-cyprindiol): balance of benefits and risks remains positive—updated prescribing advice provided. 2013. Available at: https://www.gov.uk/drug-safety-update/cyproterone-acetate-with-ethinylestradiol-co-cyprindiol-balance-of-benefits-and-risks-remains-positive (accessed June 2019)
[56] Moran LJ, Hutchison SK, Norman RJ & Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2011;16(2):CD007506. doi: 10.1002/14651858.CD007506.pub2