This content was published in 2004. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Identify knowledge gaps
- What are the symptoms of venous thromboembolism (deep vein thrombosis and pulmonary embolism)?
- What are the predisposing risk factors for the development of VTE in a surgical patient?
- What mechanical methods are available for preventing VTE in surgical patients?
Before reading on, think about how this article may help you to do your job better. The Royal Pharmaceutical Society’s areas of competence for pharmacists are listed in “Plan and record”, (available at: www.rpsgb.org/education). This article relates to “clinical pharmacy” (see appendix 4 of “Plan and record”).
Venous thromboembolism (VTE), obstruction of a vein by a blood clot, is a common term for two related disorders: deep vein thrombosis (DVT) and pulmonary embolism (PE). The most likely site of a DVTis in the deep calf veins (60 per cent), referred to as distal DVT. Less likely sites include the femoral veins (22 per cent) and popliteal veins (7.8 per cent), collectively referred to as proximal DVT.1 (see Figure 1).
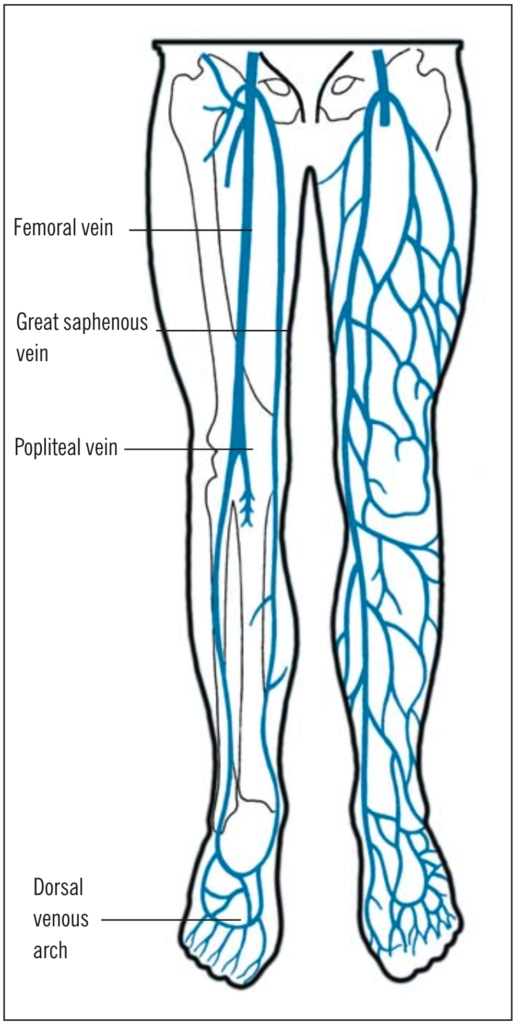
DVTs often originate at sites of vascular trauma or in areas of slow-moving or static blood (eg, around valve cusps), which trigger localised blood coagulation and thrombus formation. Thrombosis usually starts in the calf veins and can extend to the proximal veins. Distal DVTs might not be associated with major complications because they can lyse spontaneously. However, they become clinically important if they extend proximally.If the venous thrombus then breaks off and travels to, and obstructs, the arteries in the lungs it is referred to as a PE.
Signs and symptoms of DVTs and PEs
Symptoms of DVT can include pain, erythema, warmth, tenderness and abnormal swelling of the calf or thigh in the affected limb. New onset atrial fibrillation, unexplained breathlessness or cough can suggestPE. However, 90 per cent of patients diagnosed with PE can show no signs of preceding DVT.
A DVT can be diagnosed in several ways, including d-dimer tests, plethysmography (assessing the changes in volume of blood flowing through a limb) and investigations that visualise the thrombus (eg, venography, ultrasonography, spiral computed tomography and magnetic resonance imaging). PE can be investigated using radionucleotide ventilation perfusion scanning or CT pulmonary angiography. It is not routine practice to look forDVT or PE in asymptomatic surgical patients.
Predisposing factors
In 1846, German pathologist Rudolf Virchow described a triad of risk factors for VTE: stasis of the venous circulation, hyper-coagulable states and trauma to blood vessel walls. Only one of these factors is needed for thrombi formation.
Stasis of venous circulation Normally, when a person walks, the plantar venous plexus of the foot compresses. This, together with contraction of the calf muscles, propels blood from the lower extremities back towards the heart. Peri-operative immobility deprives the deep veins of the legs of the pumping action of calf muscles. Stasis of venous blood (decreased velocity of blood flow, due to venous dilation and pooling or venous obstruction), especially behind the valve cusps of deep veins, predisposes to thrombus formation. It is also purported that venous stasis inhibits the blood’s ability to carry away locally activated clotting factors — concentration rises and potentiates continued clot development.
Hypercoagulable states Hypercoagulable states alter normal blood haemostasis mechanisms.They can occur due to a range of inherited or acquired conditions. Acquired conditions include antiphospholipid syndrome, systemic lupus erythematosus and polycythaemia rubra vera. Inherited conditions include factor V Leiden mutation, protein C or S deficiency and antithrombin III deficiency. Hypercoagulability can also arise as a result of dehydration (haemoconcentration is associated with increased blood viscosity and reduced blood flow). It has been suggested that patients presenting with idiopathic VTE might have a genetic predisposition to hyper-coagulation that has remained sub-clinical until an additional risk factor (eg, immobilisation in the peri-operative period) was encountered.
Trauma Endothelial damage caused by initial trauma (eg, bone fractures or soft tissue contusions) or direct vascular damage (eg, endothelial damage during subsequent surgery) can predispose to initiation of thrombosis. Normally, the blood vessels’ endothelial lining is smooth with a negatively charged layer of surface proteins.When a vessel wall is injured, its lining loses its negative charge and becomes uneven, causing platelet adhesion and aggregation and triggering the coagulation cascade.
Microtears in vessel walls can also occur asa result of distension and venous stasis.General anaesthesia decreases vascular tone and distends veins.The state of hydration and the patient’s position during an operation will affect the amount of venous dilation that occurs.
Other predisposing factors to VTE include increasing age, obesity, drugs (eg, hormone replacement therapy, combined oral contraceptives or tamoxifen), previous history of VTE (personal and family) and venous obstruction due to external compression (eg, tumour). Malignancy is generally associated with increased risk of VTE. In most cases,VTE arises because of a combination of inherited or acquired predisposition and external circumstances. However, between 30 and40 per cent of cases appear to be idiopathic.
Risk factors in the immediate peri-operative period include:
- Nature and duration of operation
- The anaesthetic technique used
- The degree and duration of immobility (including that following discharge from hospital)
- Presence of dehydration or sepsis
Risk assessment
Despite the well documented morbidity and mortality associated with VTE, patients often receive inadequate prophylaxis. This is due either to inadvertent omission or to the use of a lower level of prophylaxis than appropriate for the level of risk. The Thromboembolic Risk Factors (THRiFT) consensus group recommends that all hospital inpatients should be assessed for VTE risk and appropriate anti-thrombotic prophylaxis administered. It suggests that hospitals should develop their own guidelines for prophylaxis of VTE using its recommendations as guidance. Guidance can also be sought from other recognised national and international organisations. Many hospitals base their thromboprophylaxis recommendations on thromboembolic risk categories of “low”, “moderate” and “high”. In practice, protocols vary in the detail of their risk stratification and proposals. Good practice should include using a validated risk assessment tool (eg, Autar DVT risk assessment). The risk of DVT should be assessed in light of the patient’s medical history, clinical signs, existing conditions (eg, varicose veins, obesity, heart failure, paralysis) and typeof surgery (nature and duration), as well as the results of any common blood tests (eg, platelet count to exclude thrombocytopenia)or specialist blood tests (eg, factor V Leiden mutation and protein C and S status) to detect coagulation abnormalities. The ScottishIntercollegiate Guidelines Network gives an indication of the percentage increase in risk of developing VTE for a range of risk factors.Following risk assessment, the most appropriate form of prophylaxis can be selected ac-cording to local recommendations. Extended DVT prophylaxis can be continued after dis-charge if the patient is not fully mobile or still considered at risk for other reasons.
Epidemiology
The incidence of deep vein thrombosis is one or two people per 1,000 general population per year.Post-operative VTE represents a serious threat to patients who have undergone a surgical procedure. Without prophylaxis, the mean frequency of all DVTs ranges from 9 per cent following transurethral prostatectomy to over 50per cent in major orthopaedic procedures and PE rates range from 1.6 per cent in general surgery to6.9 per cent following traumatic orthopaedic surgery. The incidence of fatal PE ranges from0.87 per cent in general surgery to 4 per cent inpatients with a fractured femur neck.
VTE has a recurrence rate of approximately 7 per cent at six months and death occurs in about6 per cent of patients with DVT and 12 per cent of patients with PE within one month of diagnosis.2 There is also substantial morbidity due to post-thrombotic syndrome (ie, leg pain, venous ulceration or eczema), affecting about 10 per cent of patients with symptomatic DVTs within five years3 and between 20 and 30 per cent of patients within 13 years of an acute DVT.4
Although the risk of developing VTE after surgery has fallen over the past decades, the cost of treating VTE-related events remains high. For example, a quarter of venous ulcer cases are attributed to previous DVT and cost the NHS about £400m each year.5
Prophylaxis of thromboembolism
In the absence of prophylaxis the development of DVT or PE is common. DevelopingVTE complications is most likely in the first few days after an operation. The most efficient way to prevent VTE is to use routine prophylaxis for all moderate- to high-risk patients.
For patients undergoing surgery, a triple regimen (unless contraindicated) should be adopted. This would include mechanical methods of improving blood flow, pharmacological therapy and other general measures, such as hydration and pre- and post-operative physiotherapy, to decrease coagulability. Immobility increases DVT risk by 10-fold, so early mobilisation and leg exercises must been encouraged.
It is beyond the scope of this article to analyse critically the differences in efficacy of the various methods used.
Mechanical methods
Mechanical methods prevent VTEs by increasing mean blood flow in leg veins, so reducing venous stasis.Their advantage is that they do not increase the risk of bleeding so they are beneficial in patients in whom antithrombotic drugs should be avoided. Mechanical “devices” include:
- Graduated elastic compression stockings(GECS)
- Intermittent pneumatic compression(IPC) devices, such as uniform or sequential compression devices
- Mechanical foot pumps
- Inferior vena cava filters
Graduated elastic compression stockings Graduated compression involves applying constant forces to a limb, with high levels of compression at some sites and lower ones at others. GECS (eg, Thromboembolism Deterrent [TED] stockings) are widely used in the UK. If properly fitted, they promote efficient emptying of leg veins, hence reducing stasis. The application of graduated circumferential pressure from distal to proximal areas, in combination with normal muscular activity in the limb, is thought to displace blood from the superficial to the deep venous system via the perforating veins and proximally out of the limb. Raised venous pressure in the legs increases the velocity of flow in the deep system. However, if not properly sized, GECS can compress arteries as well, leadin gto impaired circulation.
GECS also reduce the diameter of veins and bring valve cusps into opposition, restoring patency and preventing venous reflux. The reduction in venous distention reduces the occurrence of microtears in the endothelium. Clinical data presented by TycoHealthcare suggest that TED stockings will reduce DVT occurence by 68 per cent.
Below-the-knee stockings can be more comfortable for patients with swollen or arthritic knees and are easier for patients to put on without assistance. They can also be useful in patients who are too large or small for optimum full leg stocking fit and those having surgery on the upper part of the leg. However, SIGN recommends that, where possible, thigh-length stockings should be used rather than knee-length stockings (eventhough patients find knee-length stockings more acceptable) because a substantial number of DVTs develop above the knee.
In order to avoid trauma and discomfort, and to achieve optimum prophylaxis, attention must be given to:
- Correct sizing and fitting according to manufacturer’s instructions
- Checking the fit daily (because leg circumference can change)
- Making sure the stocking does not roll down (to avoid a torniquet effect — this can impede venous return and increase venous pooling and clot formation)
- Not removing stockings for more than 30minutes per day
Data also suggest that the combination of TED stockings with heparin results in a greater reduction in the incidence of DVT compared with heparin alone. The use ofGECS with pharmacological prophylaxis orIPC in surgical patients addresses all three aspects of Virchow’s triad. That is, increased velocity of venous return (so reducing stasis),stimulation of regional fibrinolytic activity(so reducing hypercoagulability) and reduction of venodilation (so reducing endothelialdamage).
Contraindications for using stockings include:
- Local skin conditions (eg, dermatitis, recent skin graft)
- Severe ischaemia or peripheral vascular disease (to prevent further reduction in arterial circulation)■Peripheral neuropathy
- Marked leg or pulmonary oedema ■Extreme leg deformity
- Known sensitivity to material of stocking
Intermittent pneumatic compression devices IPC devices consist of an inflatable garment for the limb and an electrical pneumatic pump that fills the garment with compressed air. The garment is inflated and deflated, periodically compressing the calf or thigh muscles (or both)circumferentially (the pressure chamber wraps around the leg) and stimulating fibrinolysis. Cycle times and pressures vary.
First, compression pushes blood from superficial veins into deep veins and increases the velocity of flow into the femoral veins.This decreases venous stasis, blood pooling and venous congestion. It also enhances endogenous fibrinolytic activity.
IPC devices are particularly useful in the immediate peri-operative period, but are not tolerated by some patients because they are somewhat cumbersome. It might not be practical to use IPCs in patients with fractures, plaster casts or external fixation devices.Patient education about device function and extra features (eg, cooling systems) can improve compliance.IPC devices offer uniform or sequential compression. Sequential compression devices send waves of compression up the leg and appear to be more effective than uniform compression in preventing DVTs. The Flowtron Excel system is an example of a uniform compression device.
It has been reported that sequential graded compression provides a greater and more consistent velocity of venous blood flow than uniform compression. A sequential device achieves better emptying of the vasculature than the uniform device and maximises venous valve cusp clearance.The SCD Response device has circumferential, sequential, graduated (highest pressure at ankle, medium pressure at the calf and lowest pressure at the thigh) compression as well as automated and personalised settings.
Mechanical foot pumps A mechanical foot pump is designed to overcome stasis of venous blood. When worn, it flattens the metatarsal arch and empties the plantar venous plexus, reproducing the effect of normal weight bearing. Mechanical foot pumps are effective for prophylaxis of DVT and are often used for orthopaedic patients, especially in cases where IPC devices or stockings cannot be used.
Foot compression devices cannot constrict blood flow at the top of the calf, thus potentially offering better vessel emptying. Patient compliance is improved by comfort and ease of use. Foot pumps also decrease vessel distension, lowering risk of injury to the endothelial lining. By increasing blood velocity, they assist in moving locally activated clotting factors as well as enhancing fibrinolysis. They are particularly useful in trauma cases and severely obese patients.
Different foot pumps use different speeds and pressures. Foot impulse technology (eg, used by the A-V Impulse System) has been recommended for prophylaxis in elective hip replacement, by an international consensus statement.5
Inferior vena cava filters Inferior vena cava filters are inserted percutaneously under x-ray guidance, usually via the femoral vein, and present a physical barrier to emboli.They are useful where anticoagulation is contraindicated or in patients who develop recurrent PEs despite adequate anticoagulation. Filters are also used in patients at high risk of proximal DVT extension or pulmonary embolism.Inferior vena cava filters prevent PE, but do not halt the thrombotic process or prevent venous thrombosis.
Action: practice points
Reading is only one way to undertake CPD and theSociety will expect to see various approaches in a pharmacist’s CPD portfolio.
- Try to put on a pair of full length graduated compression stockings, according to the manufacturer’s instructions.
- Find out more about the IPC devices and foot pumps used in your hospital.
- Revise the anatomy of the leg (bones, muscles and vasculature). For example, visitwww.venous-info.com
Evaluate
For your work to be presented as CPD, you need to evaluate your reading and any other activities.Answer the following questions: What have you learnt? How has it added value to your practice? (Have you applied this learning or had any feedback?) What will you do now and how will this be achieved?
ACKNOWLEDGEMENT
We are grateful to Tyco Healthcare and Novamedix for providing references and advice about their devices for preventing VTE.
References
- Wallis M, Autar R. Deep vein thrombosis: clinical nursing management. Nursing Standard 2001;15:47–54.
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107:i1–i8.
- Kearon C. Natural history of venous thromboembolism. Circulation 2003;107:i22–i30.
- Hawkins DW. Recent advances in the prophylaxis of venousthromboembolism: modulating the coagulation cascade. American Journal of Health-System Pharmacists 2001;58:S2–3.
- Nicolaides A (editor). Prevention of Venous Thromboembolism: International Consensus Statement 2002. Available at: www.jvascbr.com.br (accessed 11October 2004).
Resources
- A recent article published in the BMJ, explains various screening and diagnostic methods for VTE, as well as treatment. See Tovey C, Wyatt S. Diagnosis, investigation and management of deep vein thrombosis. BMJ2003;326:1180–4.
- Scottish Intercollegiate Guidelines Network. Prophylaxis of venous thromboembolism: a national clinical guideline 2002.Available at www.sign.ac.uk (accessed 28 September 2004).
- The THRiFT II statement stratifies patients according to their clinical circumstances and underlying factors. It lists a wide range of thromboembolic risk factors and offers thromboprophylaxis recommendations based on risk category. (See Risk of and prophylaxis for venous thromboembolism in hospital patients. Phlebology;13:87–97.)
- A report from the American College of Chest Physicians’ 7th conference (Antithrombotic and thrombolytic therapy, available at: www.chestjournal.org) discusses rationale and risk factor stratification for prophylaxis of VTE.


