Mclean/Science Photo Library
In June 2019, Simon Stevens, chief executive of NHS England, described ‘tumour agnostic’ drugs as “game-changing” and announced the NHS’s desire to fast-track their approval for people with cancer
[1]
.
Yet, just seven months later, the National Institute for Health and Care Excellence (NICE) released draft guidance which did not recommend larotrectinib — the first medicine specifically developed to target a mutation rather than a particular tumour type to be licensed in Europe[2]
,[3]
.
This is different to our current approach where cancer therapies target the tumour type
This is not the first time that an expensive cancer drug has fallen foul of NICE. Of course, NICE’s mission is to determine the cost–benefit ratio of treatments for the NHS, and the high price for larotrectinib may be a factor, but its decision also stems from the very reason larotrectinib appears to be so exciting: the drug was developed to target a rare mutation.
“This is different to our current approach where cancer therapies target the tumour type,” says Lord Ara Darzi, giving the example of drugs that are licensed to treat breast or colon cancer. Lord Darzi chairs the Accelerated Access Collaborative (AAC), a medical innovation partnership which, he says, aims “to get more proven innovations into the hands of clinicians and benefitting patients, faster”[4]
. Along with NHS England, one of its partners, the AAC has signalled that tumour agnostic drugs are one of its priorities.
Some working in the field, including pharmaceutical companies such as Bayer — the manufacturer of larotrectinib — have indicated that the problem may be that NICE does not currently have the right methods to evaluate these new drugs. Others think that there is simply not enough data and that only time can solve this. Whatever the issue, this is certain: tumour agnostic drugs are challenging not only the way we think about cancer treatment, but the way we think about diagnosis and even clinical trial design.
Ground-breaking drugs
Melanie Dalby, a pharmacist who currently works at Barts Health NHS Trust as a Macmillan patient experience and engagement lead, has worked with cancer patients for almost a decade. She recalls the introduction of two drugs — pembrolizumab and nivolumab — as “ground-breaking”.
Both drugs are immunotherapies that help the immune system to identify and fight cancer cells. Tumour cells can evade the body’s immune system by turning off T-cells at an ‘immune checkpoint’ receptor. Immunotherapies bind to and block this receptor, allowing the immune system to mount an attack. However, one of the main ways in which these drugs have broken the mould is that, despite being initially approved for specific cancers, they are now licensed for use in any tumour that has certain biomarkers (see Figure).
Matthew Krebs, a clinical senior lecturer in experimental cancer medicine at the University of Manchester, cites the 2017 decision on pembrolizumab by the US Food and Drug Administration (FDA): “That was the first example of a drug being approved that was agnostic to disease type and based on a molecular finding”.
If you take the brakes off the immune system by giving immunotherapy, your immune system is more likely to recognise those cancer cells
Pembrolizumab, it turns out, is particularly good at tackling tumours with a high-degree of microsatellite instability — something that “occurs in various different cancer types, but at low-frequency,” Krebs explains.
Microsatellite instability is when the process of DNA repair goes ‘haywire’, leading to a large number of genetic mutations. This causes a lot of neoantigens — new proteins that the immune system has never encountered before — to appear on the surface of cancer cells.
“If you take the brakes off the immune system by giving immunotherapy, your immune system is more likely to recognise those cancer cells [as foreign],” Krebs explains.
Pembrolizumab and nivolumab were developed as a sort of ‘next generation’ of immunotherapy treatments, Krebs explains. They were then tested in patients with several different cancers and approved on a cancer-type-specific basis (see Figure).
As we’ve learned more about these drugs, he adds, it has become clear that patients who have a high number of mutations — whatever the cancer type — are the ones who are more likely to benefit.
Thus, pembrolizumab and nivolumab opened the door for a new way of thinking about cancer medicines and raised questions as to whether some targeted drugs could work in all cancers with a particular biomarker, regardless of their tissue of origin.
However, this is not necessarily the case: Krebs cites the example of BRAF inhibitors, which work well for melanoma patients with BRAF mutations, but do not seem to have a similar effect in colorectal cancer patients with the same mutation. Researchers are still trying to figure out why this is, with the theory being that one biomarker may cancel out another “which means [a drug] might work for one patient, but not another”, Dalby explains.
“We just don’t know because we don’t know all of the biomarkers out there.”
Nonetheless, the success of pembrolizumab and nivolumab galvanised researchers and drug companies to continue their search for these agents, which is where both larotrectinib and entrectinib — another tumour agnostic drug currently being assessed by NICE — come in. These drugs have been specifically developed to target the neurotrophic tyrosine receptor kinase (NTRK) fusion mutation – which can lead to abnormal proteins that may cause cancer cells to grow – in whatever tumour it may appear.
So far, these agents look promising, with one study of larotrectinib finding that 75% of patients with the NTRK fusion mutation across 17 different tumour types responded to the drug[5]
. In the past two years, both larotrectinib and entrectinib have been granted accelerated approval by the FDA for use in patients with any solid tumours that harbour the NTRK fusion mutation.
“This genetic change is found across potentially all different solid tumour cancer types, but it can be particularly prevalent in some rarer cancers” explains Duncan Sim, a policy advisor for Cancer Research UK.
This means that the small number of patients who develop certain rare cancers, such as secretory carcinoma of the breast or salivary gland, and infantile fibrosarcoma, are likely to have the NTRK mutation and benefit from these drugs. But, a very small percentage of patients with other solid tumours — wherever they originate — will also have this mutation[6]
.
This leads to the question: how do we find all the patients who may benefit from this treatment?
The NHS is doing a lot of thinking about how it can offer the right genetic testing to patients right across the country
“You have to be much more broad-brush about the number of patients that you offer this test to,” Sim says. But that can be expensive and difficult to organise.
“I don’t think we have any sort of national steer as to what we should be testing,” Dalby adds.
Yet that may be about to change. “The NHS is doing a lot of thinking about how it can offer the right genetic testing to patients right across the country,” Sim says. The idea is that a new NHS Genomic Medicine Service (GMS) will coordinate testing through seven ‘laboratory hubs’ spread throughout the country. The GMS will drive more personalised treatments and interventions, leading to the routine use of whole genome sequencing, along with newer genomic technologies.
“[This] will transform current testing pathways and help facilitate access for patients to new, innovative treatments,” Lord Darzi explains.
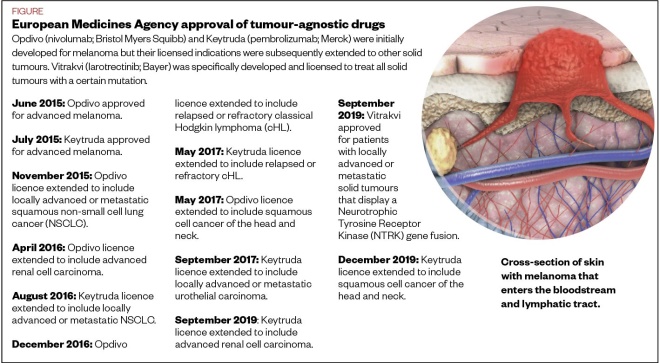
Figure: European Medicines Agency approval of tumour-agnostic drugs
Source: European Medicines Agency
Out with the old
Traditional cytotoxic chemotherapy — the kind of treatment that is lodged in the popular imagination when we talk about cancer — targets all fast-growing cells, “which are your cancer cells, but also things like your hair, your skin and your gut,” Dalby says. They also disrupt the production of new red and white blood cells, leading to anaemia and potentially life-threatening depletion of immune cells.
The promise of tumour agnostic drugs is really the same promise as that of all targeted cancer drugs — that they will be more specific and thus more effective, with fewer side effects. This means that “not only do you need a good target, you also need to have a good drug that’s as selective as possible for the pathway you’re trying to inhibit,” Krebs says.
Of course, tumour agnostic drugs are not free of side effects, but they tend to be better-tolerated than cytotoxic treatment, Krebs explains. They also do not eliminate the possibility of the cancer developing resistance; although, because these drugs are so new on the scene, Dalby says: “I don’t know if we are quite there in terms of treating somebody, them being in remission, [and then] someone relapsing.”
The lack of data on larotrectinib is one of the reasons NICE did not approve it.
Jacoline Bouvy, a senior scientific advisor for NICE, says that the issue with tumour agnostic drugs “is more a question of when they are evaluated, than how they are evaluated”. Part of that is down to the rarity of the mutation and thus the small number of patients being treated (the study on larotrectinib cited above was in 55 patients[5]
). This also means that a randomised controlled trial becomes impossible — there are simply not enough people for both a treatment and a control arm. “It just isn’t doable,” says Krebs.
Furthermore, where drugs are targeting a novel biomarker, such as with larotrectinib, we may not know enough about the significance of that biomarker. “Do patients with NTRK-positive lung cancer have a similar, better or worse prognosis than patients with NTRK-negative lung cancer?” Bouvy asks.
Randomised controlled trials are no longer feasible for these kinds of drugs … we have to think of new ways of addressing this
Assessing the efficacy of these drugs means measuring their effectiveness against multiple different cancer types when, traditionally, drugs are measured by their effects on one cancer type.
“It’s such a different way of doing things for NICE,” Sim says.
“That model [randomised controlled trials] is no longer feasible for these kinds of drugs. And we have to think of new ways of addressing this,” adds Krebs.
NICE has indicated that the cancer drugs fund, which allows for a period of managed access to certain drugs on the NHS, may be “a suitable route” for getting larotrectinib and entrectinib to patients while additional data are collected, Bouvy says[7]
. These data will come both from clinical trials and so-called ‘real-world data’ gathered from patients’ experiences within healthcare systems in the UK and abroad.
Krebs believes using such real-world data to evaluate tumour agnostic drugs is essential.
“In the United States, for example, where they’ve been doing molecular testing for a long time, there are databases of patients who have the NTRK fusion [mutation],” he says. This means you can look up those patients and check what standard care treatments they received and how they got on with them, and then you can compare this to how the same patient groups respond in trials to the targeted drugs.
“Then you have a sort of artificial control arm,” Krebs points out.
NICE has also said that it is hoping that Bayer will reduce the price of larotrectinib, which costs £15,000 for a 30-day supply[2]
. “Nothing has been taken off the table and things are still progressing,” Sim says. In the meantime, NICE has suggested that entrectinib may beat larotrectinib to be the first tumour agnostic drug to be recommended for use on the NHS, provided it receives its marketing authorisation. The organisation has said that there are “constructive conversations” happening with its manufacturer, Roche, on a commercial deal.
The drugs are likely to have a lasting legacy in changing the way the NHS treats cancer patients
Ultimately, given the rarity of the NTRK fusion, larotrectinib and entrectinib are unlikely to benefit huge numbers of patients. Nonetheless, the drugs are likely to have a lasting legacy in changing the way the NHS treats cancer patients. Lord Darzi suggests that tumour agnostic drugs represent an opportunity for the NHS “to pioneer a new class of cancer therapy, embedding diagnostic provision and the robust translation of genomic provision into new clinical practice”.
Furthermore, he believes that the NHS may need to move away from a system in which patients are treated “by clinicians who are expert in specific tumour sites” to “a multidisciplinary approach”.
Krebs also argues that patients would benefit from accessing early and broad genetic screening and points out that as this service is scaled up, costs will come down.
Moving down the line there will also be new ways of characterising tumours further, and new, targeted drugs, says Krebs.
“All these things are going to become more and more important in terms of how we personalise treatments for patients.”
References
[1] NHS England. 2019. Available at: https://www.england.nhs.uk/2019/06/fast-track-game-changing-cancer-drugs/ (accessed March 2020)
[2] National Institute for Health and Care Excellence. 2020. Available at: https://www.nice.org.uk/news/article/nice-encourages-further-data-collection-on-game-changing-histology-independent-cancer-drugs (accessed March 2020)
[3] National Institute for Health and Care Excellence. 2020. Available at: https://www.nice.org.uk/guidance/GID-TA10229/documents/129 (accessed March 2020)
[4] National Institute for Health and Care Excellence. 2020. Available at: https://www.nice.org.uk/aac (accessed March 2020)
[5] Drilon A, Laetsch T, Kummar S, et al. N Engl J Med 2018;378(8):731-739. doi: 10.1056/NEJMoa1714448
[6] Ma P. HemOnc Today online. 2019. Available at: https://www.healio.com/hematology-oncology/lung-cancer/news/print/hemonc-today/%7B912d7a1c-ab0a-4116-962a-0401ad8e7a2c%7D/cancer-agnostic-ntrk-inhibitor-approval-ushers-in-paradigm-of-personalized-genomics-guided-therapeutics (accessed March 2020)
[7] NHS England. 2020. Available at: https://www.england.nhs.uk/cancer/cdf/ (accessed March 2020)