Download the full print version of the infographic here.
What are macrolides?
- Macrolides are broad spectrum antibiotics that are typically used as an alternative to penicillin in allergic patients
- Macrolides accounted for 15.8% of antibiotics prescribed in primary and secondary care in England in 2018
- Macrolides are associated with an increased risk of major birth defects when taken during the first trimester of pregnancy, compared with penicillin (27.65/1,000 vs 17.65/1,000), so should be used with caution in pregnant women
- Macrolides increase risk of QT interval prolongation, especially in older people, those with cardiac risk factors, and those receiving other medicines that prolong the QT interval, so should be used with caution in these patient groups
How they work
Macrolides exert their effects by blocking bacterial protein synthesis.
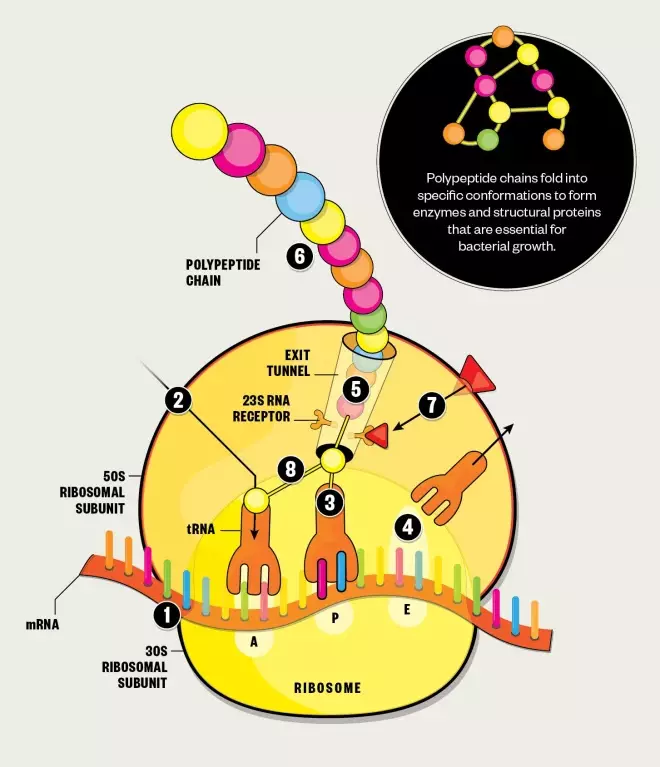
Protein synthesis
1. The ribosome moves along the messenger RNA (mRNA), reading the nucleotide sequence and recruiting transfer RNA (tRNA) that carry the correct amino acids.
2. The ribosome has three tRNA binding sites: aminoacyl (A), peptidyl (P) and exit (E), which the tRNA pass through during protein synthesis.
3. When tRNA is in binding sites A and P, the ribosome links together their amino acids by catalysing peptide bond formation, as instructed by the codons of the mRNA.
4. The deacylated tRNA then moves to the E binding site, ready for ejection from the ribosome.
5. As more amino acids are linked together, a polypeptide chain is formed.
6. When the ribosome encounters a stop codon on the mRNA, protein synthesis is terminated and the polypeptide chain is released from the ribosome via the exit tunnel.
Antibiotic effect
7. Macrolides gain entry into the cell by diffusing across the lipid bilayer. They bind to the 23S ribosomal RNA (23S rRNA) receptor within the exit tunnel of the ribosomal 50S subunit, blocking the tunnel and disrupting protein formation.
8. Macrolides also disrupt protein formation by preventing the ribosome from catalysing peptide bond formation.
Developing resistance
The first reports of resistance to macrolides came just four years after they had entered clinical practice. Resistance genes can be passed on during bacterial replication, or can transfer between bacteria via plasmids or other mobile genetic elements. Genes for macrolide resistance act via three main mechanisms — target site modification, activation of efflux pumps and enzymatic modification of the drug.
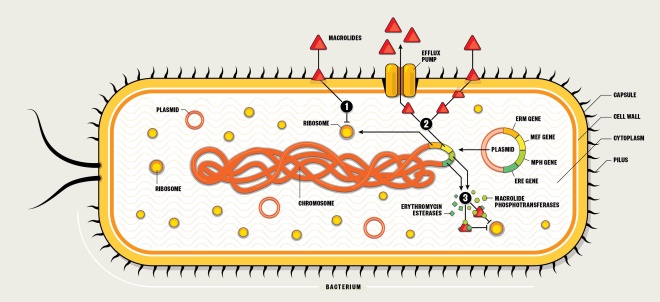
1. Target site modification
- Erythromycin ribosome methylation (erm) genes (e.g. erm A, B, C) are transcribed and translated to produce enzymes that can methylate the 23S rRNA binding site on the 50S subunit.
- The altered binding site results in decreased binding affinity of macrolides.
- This results in high-level resistance.
2. Efflux pump activation
- Macrolide efflux (mef) genes (e.g. mef A, B, E) are transcribed and translated to produce transporters that pump out macrolides before they can reach their site of action on the 50S ribosomal subunit.
- This results in relatively low to moderate resistance.
3. Enzymatic modification
- Macrolide phosphotransferase (mph) and erythromycin esterase (ere) genes are transcribed and translated to produce enzymes that can modify macrolides so they are no longer capable of binding effectively to the 50S ribosome.
- The altered binding means the macrolides are unable to exert an effect.
- This results in high-level resistance.
Macrolide use is decreasing…
In line with other antibiotics, macrolide prescribing in primary care in England has decreased over the past 5 years, falling by 17.3% from 4.2 milllion items in 2015/2016 to 3.5 million items in 2019/2020.
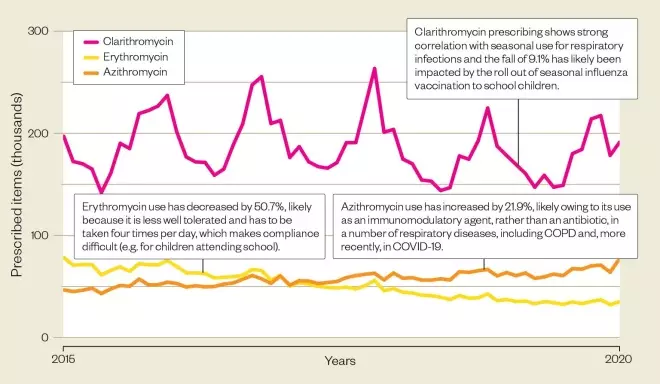
Source: Open Prescribing
…But resistant infections are increasing
Macrolide resistance in Gram-positive infections is increasing, largely because of an increasing incidence of infections. Higher numbers of resistant infections are seen in older age groups.
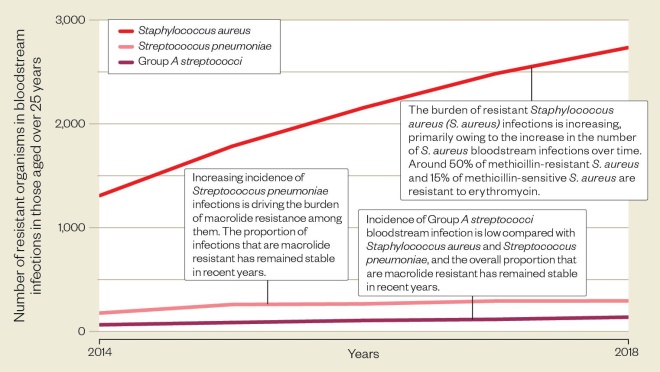
Source: ESPAUR report 2018-2019, Public Health England
Antimicrobial stewardship
Risk of antimicrobial resistance can be reduced by taking the following steps:
- Do not start antimicrobial therapy unless there is clear evidence of infection
- Take a thorough drug allergy history
- Avoid inappropriate use of broad-spectrum antibiotics
- Comply with local antimicrobial prescribing guidance
- Document clinical indication on drug chart and in clinical notes
- Include review/stop date or duration
- Obtain cultures prior to commencing therapy where possible (but do not delay therapy)
- Review continuing need for antibiotics at 48–72 hours and document a clear action plan
- Provide self-care advice to patients with self-limiting or viral infections and recommend appropriate symptom relief, minor ailment treatments or referral where necessary
References
Sources: British National Formulary; BMJ 2020;368:m331; Pharmazie 2010;65:631; Eur J Med Chem 2012;47:462; Front Microbiol 2018;9:1942; OpenPrescribing; National Institute for Health and Care Excellence; Public Health England.
Illustration: Alisdair MacDonald
Editorial advisers: Ting Yee Yau, highly specialist pharmacist, anti-infective and infection management, St George’s University Hospitals NHS Foundation Trust. Diane Ashiru-Oredope, lead pharmacist, Healthcare-associated infections & antimicrobial resistance division (HCAI & AMR), Public Health England. Elizabeth Beech, national project lead, AMR, NHS England and NHS Improvement. Rebecca Guy, senior scientist, HCAI & AMR division, Public Health England. Philip Howard, consultant antimicrobial pharmacist, Leeds Teaching Hospitals NHS Trust. Peter Taylor, professor of microbiology, UCL School of Pharmacy.