Open access article
The Royal Pharmaceutical Society has made this article free to access in order to help healthcare professionals stay informed about an issue of national importance.
To learn more about coronavirus, please visit: https://www.rpharms.com/resources/pharmacy-guides/wuhan-novel-coronavirus
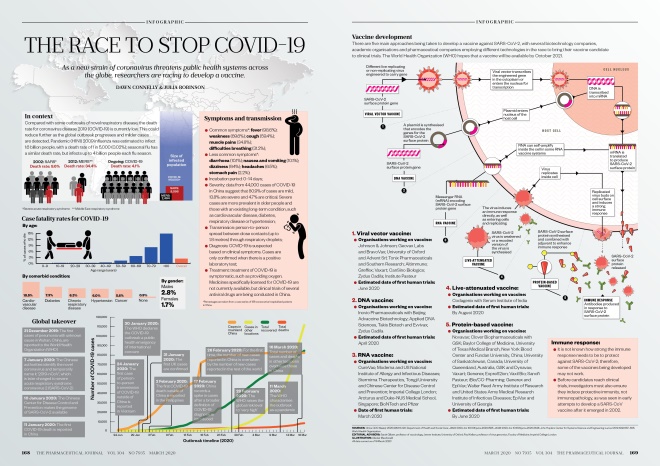
Source: Alasdair Macdonald
Download the full print version of the infographic here.
In context
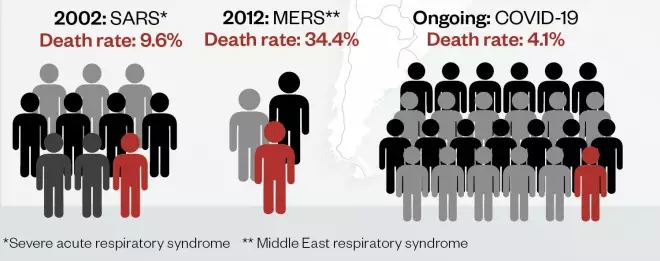
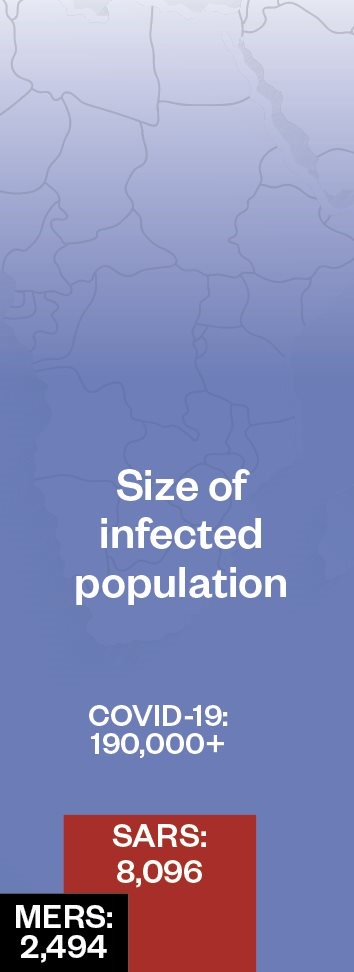
Compared with some outbreaks of novel respiratory disease, the death rate for coronavirus disease 2019 (COVID-19) is currently low. This could reduce further as the global outbreak progresses and milder cases are detected. Pandemic (H1N1) 2009 influenza was estimated to infect 1.6 billion people, with a death rate of 1 in 5,000 (0.02%); seasonal flu has a similar death rate, but infects up to 4 billion people each flu season.
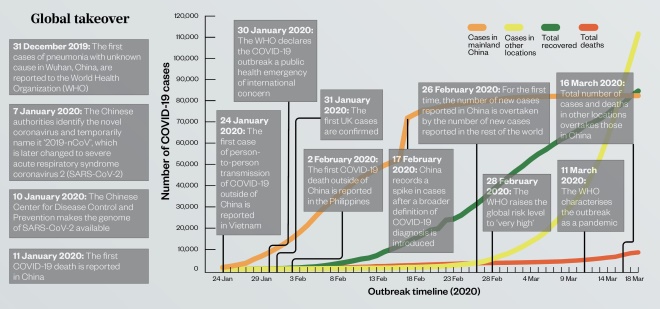
Global takeover
Symptoms and transmission
- Common symptoms*: fever (98.6%); weakness (69.6%); cough (59.4%); muscle pains (34.8%); difficulties breathing (31.2%);
- Less common symptoms*: diarrhoea (10.1%); nausea and vomiting (10.1%); dizziness (9.4%); headaches (6.5%); stomach pain (2.2%);
- Incubation period: 0–14 days;
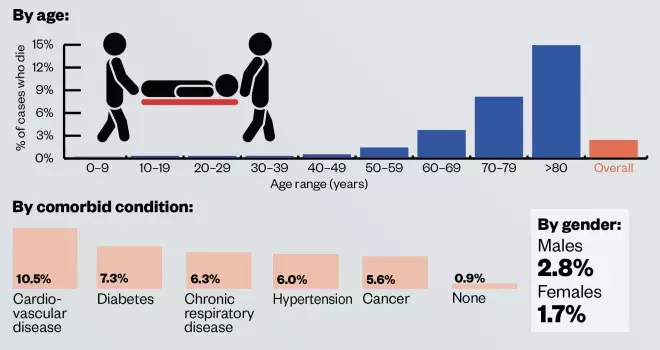
- Severity: data from 44,000 cases of COVID-19 in China suggest that 80.9% of cases are mild, 13.8% are severe and 4.7% are critical. Severe cases are more prevalent in older people and those with an existing long-term condition, such as cardiovascular disease, diabetes, respiratory disease or hypertension;
- Transmission: person-to-person spread between close contacts (up to 1.8 metres) through respiratory droplets;
- Diagnosis: COVID-19 is suspected based on clinical symptoms. Cases are only confirmed when there is a positive laboratory test;
- Treatment: treatment of COVID-19 is symptomatic, such as providing oxygen. Medicines specifically licensed for COVID-19 are not currently available, but clinical trials of several antiviral drugs are being conducted in China.
*Percentages are taken from a case series of 138 consecutive hospitalised patients in China.
Vaccine development
There are five main approaches being taken to develop a vaccine against SARS-CoV-2, with several biotechnology companies, academic organisations and pharmaceutical companies employing different technologies in the race to bring their vaccine candidate to clinical trials. The World Health Organization hopes that a vaccine will be available by October 2021.
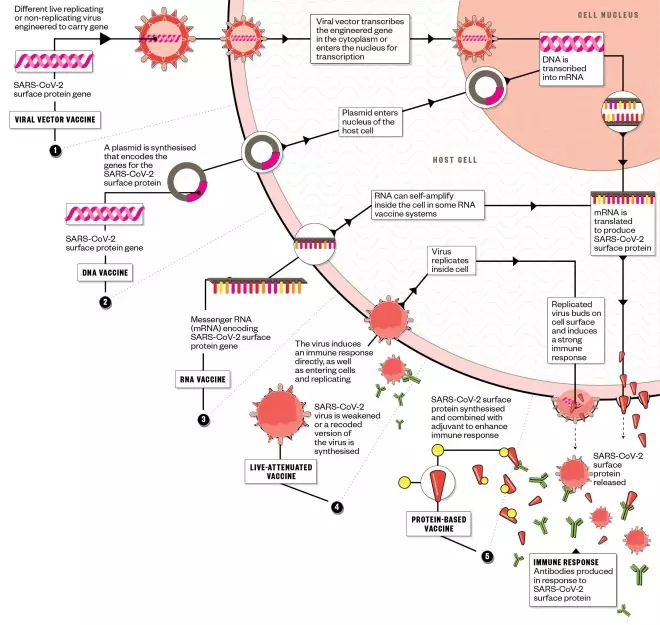
1. Viral vector vaccine:
- Organisations working on vaccine:
Johnson & Johnson; Geovax Labs and BravoVax; University of Oxford and Advent Srl; Tonix Pharmaceuticals and Southern Research; Altimmune; Greffex; Vaxart; CanSino Biologics; Zydus Cadila; Institute Pasteur
- Estimated date of first human trials: June 2020
2. DNA vaccine:
- Organisations working on vaccine:
Inovio Pharmaceuticals with Beijing Advaccine Biotechnology; Applied DNA Sciences, Takis Biotech and Evvivax; Zydus Cadila
- Estimated date of first human trials: April 2020
3. RNA vaccine:
- Organisations working on vaccine:
CureVac; Moderna and US National Institute of Allergy and Infectious Diseases; Stermirna Therapeutics, Tongji University and Chinese Center for Disease Control and Prevention; Imperial College London
- Date of first human trials: March 2020
4. Live-attenuated vaccine:
- Organisations working on vaccine:
Codagenix with Serum Institute of India
- Estimated date of first human trials: By August 2020
5. Protein-based vaccine:
- Organisations working on vaccine:
Novavax; Clover Biopharmaceuticals with GSK; Baylor College of Medicine, University of Texas Medical Branch, New York Blood Center and Fundan University, China; University of Saskatchewan, Canada; University of Queensland, Australia, and Dynavax; Vaxart; Generex; ExpreS2ion; Vaxil Bio; Sanofi Pasteur; iBio/CC-Pharming
- Estimated date of first human trials: By June 2020
Immune response:
- It is not known how strong the immune response needs to be to protect against SARS-CoV-2; therefore, some of the vaccines being developed may not work;
- Before candidates reach clinical trials, investigators must also ensure they induce protective immunity, not immunopathology, as was seen in early attempts to develop a SARS-CoV vaccine after it emerged in 2002.
References
Sources: China CDC Weekly 2020;2(8):113-122; Department of Health and Social Care; JAMA 2020, doi: 10.1001/jama.2020.1585; JAMA 2020, doi:10.1001/jama.2020.2648; John Hopkins Center for Systems Science and Engineering; Lancet 2012;12(9):687–695; World Health Organization
Editorial advisers: Sarah Gilbert, professor of vaccinology, Jenner Institute, University of Oxford; Paul Kellam, professor of virus genomics, Faculty of Medicine, Imperial College London
Illustration: Alisdair Macdonald
All data correct as of 19 March 2020