Jonathan Buisson
More than 50% of pharmacies in the UK are not expected to meet the Falsified Medicines Directive (FMD) deadline, the body running the system in the UK has said.
Jerome Bertin, general manager of SecurMed, told The Pharmaceutical Journal that current information on registrations to the UK National Medicines Verification System, which will hold the data from FMD-compliant medicine packs, indicated that “less than half” of UK pharmacies would have registered, and had their credentials issued, by the deadline of 9 February 2019.
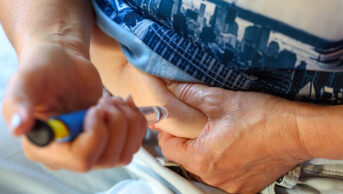
Under the Europe-wide directive, UK pharmacies are required to register with SecurMed and have the hardware in place to scan the 2D barcode that must feature on all new packs of prescription medicine sold in Europe.
The Medicines and Healthcare products Regulatory Agency (MHRA) will be responsible for enforcing FMD compliance, which could include criminal prosecution for serious and persistent breaches.
A spokesperson for the MHRA said: “We appreciate the uncertainty facing organisations due to Brexit and have worked closely with a wide range of stakeholders, including community pharmacy, to support this stakeholder-led implementation model. We have been working closely with the UK FMD working group for community pharmacy, who have published information and guidance for pharmacy contractors.”
They added that the MHRA and other regulators would “take a pragmatic approach, working with stakeholders to help them achieve compliance”.
Claire Bryce Smith, director for insight, intelligence and inspections at the General Pharmaceutical Council (GPhC), which will monitor pharmacies’ FMD compliance during its routine inspections, said that “in our discussions with pharmacy owners and pharmacy teams we have said that we do not see the implementation date of 9 February [2019] as being a ‘cliff edge’.
“If, during an inspection in the following months, we identified a pharmacy that was not meeting all of the requirements relating to FMD, we would be looking to understand the reasons why and seeking evidence that there was a clear plan in place.”
Gino Martini, chief scientist at the Royal Pharmaceutical Society (RPS), said the current lack of take-up “is likely to be a Brexit effect: people are waiting to see what happens”.
“As we get more clarity about the form of our departure from the EU, uptake should increase,” he added.
In the meantime, Martini advised pharmacists seeking more information on FMD to refer to the RPS’s FMD hub.
On 29 January 2019, the UK FMD Working Group for Community Pharmacy said it was “confident that the regulators will take a pragmatic and even-handed approach to enforcement” of FMD, but advised pharmacists that they must make an effort to be compliant by 9 February 2019.
In the event of no-deal Brexit, the UK will be unable to access the European Medicines Verification System — the EU-wide data hub at the centre of FMD — and, therefore will not be able to verify and authenticate medicines.
The MHRA has said that in this case, “the legal obligation related to this would be removed for actors in the UK supply chain” but that it will “evaluate the options for a future UK falsified medicines regulatory framework, taking into account investment already made by stakeholders”.
The British Medical Association (BMA) has said the UK is “relatively well-placed” for FMD compliance compared with the rest of Europe.
In its January 2019 member update, the BMA told doctors that Italy has dispensation to introduce the scheme more slowly; Spain is showing red on the dashboard for implementation; and in France ”there is a dispute between the government and pharmacies, so nobody is currently registered”.