Superdrug
Open access article
The Royal Pharmaceutical Society has made this article free to access in order to help healthcare professionals stay informed about an issue of national importance.
To learn more about coronavirus, please visit: https://www.rpharms.com/resources/pharmacy-guides/wuhan-novel-coronavirus
Source: Superdrug
Superdrug is selling the UK’s first CE-marked COVID-19 antibody test kit
Pharmacies have been listed as a “target setting” for COVID-19 antibody tests under specifications published by the Medicines and Healthcare products Regulatory Agency (MHRA).
The target product profile, which was issued on 19 May 2020, outlines “intended use, target populations and other desired attributes” of COVID-19 antibody tests, including what the government agency considers to be “minimally acceptable” in terms of where and by whom the test can be used.
Among the “target use settings” cited in the document, the MHRA lists “clinics, pharmacies, workplaces and other non laboratory settings” as acceptable places for the tests to be carried out.
It adds that an acceptable test would be one carried out by a healthcare professional, although a test that can be used by a “person trained in operating the test kit” is listed as being desirable.
The update comes after Public Health England approved two antibody tests developed by Roche and Abbott on 14 May 2020.
Alastair Buxton, director of NHS services at the Pharmaceutical Services Negotiating Committee (PSNC), said community pharmacies “could offer a safe and convenient location for local communities and patients to access antibody tests”.
“PSNC remains in regular dialogue with NHS England and Improvement who we expect will be involved in planning any wider rollout of antibody testing,” he added.
A spokesperson for Well Pharmacy agreed that “frontline community pharmacies should be included in making this test available for the public”.
“As we’re already trusted by communities for health and wellbeing advice, it seems natural that patients and customers would look to their pharmacy as a convenient and highly accessible source for any antibody test, and indeed for any vaccine in the future,” they said.
Rowlands also said it was “currently looking at how we could be a part of the testing process while we ensure it is safe to do so”.
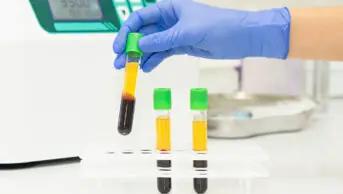
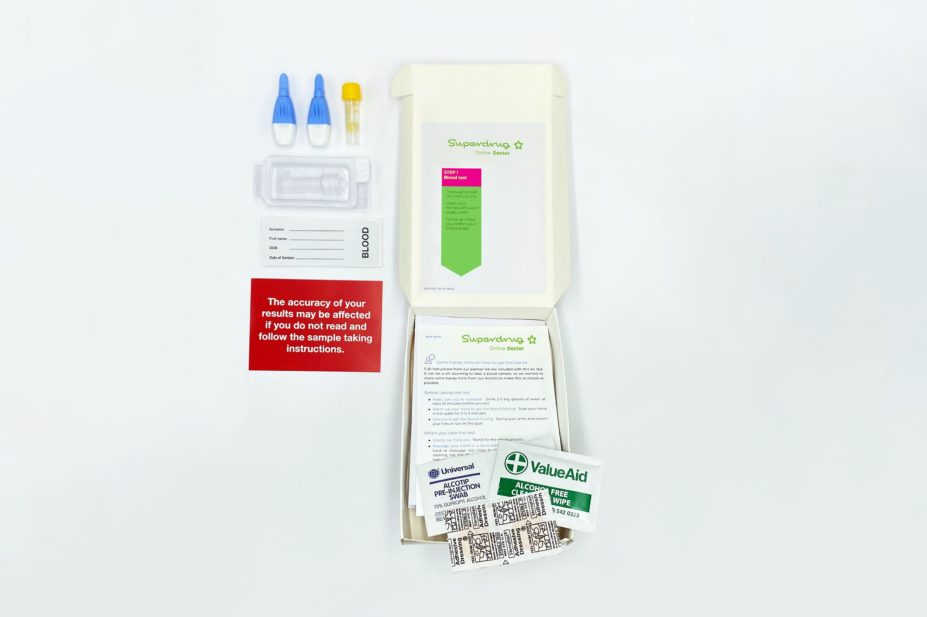
However, Superdrug is the first pharmacy to offer a CE-marked COVID-19 antibody test to the public for £69 through its online store.
The finger-prick test on sale from Superdrug, which is manufactured by Abbott and one of the tests approved by Public Health England, enables patients to collect drops of their own blood in a small vial, which is then sent to The Doctor’s Laboratory — a laboratory accredited by the UK Accreditation Service — for analysis.
A spokesperson for Superdrug told The Pharmaceutical Journal that the antibody test “provides a … serum sample to be processed on the Abbott ‘ARCHITECT’ analyser by laboratory professionals, all of whom meet Abbott’s criteria”.
The results of the test are available from Superdrug’s online pharmacy service within 24 hours of the sample reaching the lab.
Commenting on the sale of the test through Superdrug, the MHRA warned that “even if a test has a CE mark, it may have limitations”.
“For example, the link between the presence of antibodies and immunity is not proven,” they said, adding that this means “that a positive test result for antibodies does not necessarily mean that the person being tested is immune to COVID-19.”