Alamy
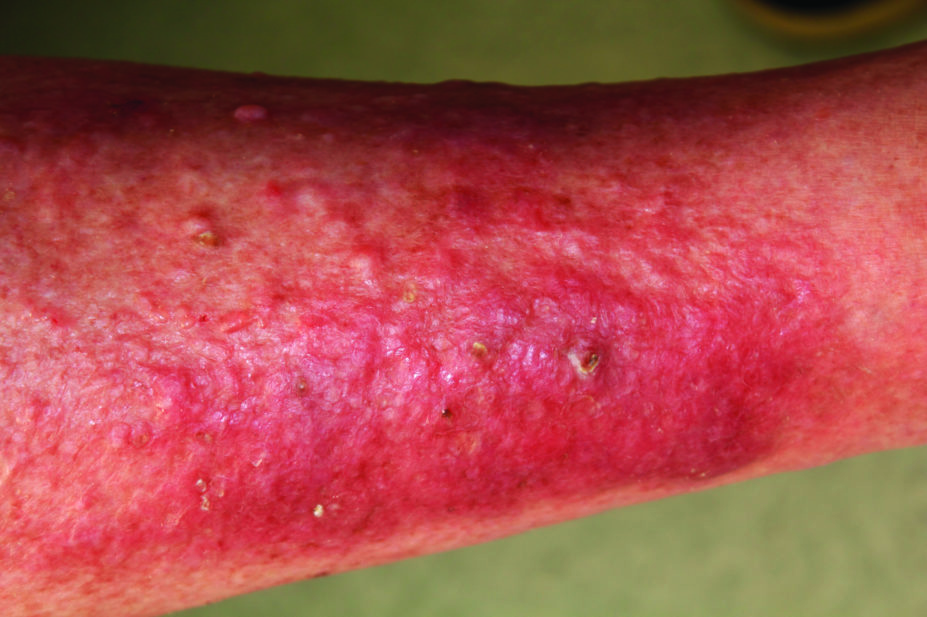
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has issued a positive opinion for dupilumab in atopic dermatitis under the Early Access to Medicines Scheme (EAMS).
The decision means that eligible patients will now be able to receive the treatment despite it not yet having a marketing authorisation in the European Union.
Dupilumab is a monoclonal antibody targeting the interleukin-4 receptor alpha, which plays a pivotal role in the signalling pathways that lead to chronic skin inflammation in atopic dermatitis.
In clinical trials involving more than 1,500 patients with moderate-to-severe atopic dermatitis, patients saw a reduction in the extent and severity of atopic dermatitis lesions, as well as reductions in itch. There were also improvements in quality-of-life measures and sleep, which is often affected by the disease.
Patients receiving dupilumab were more likely to develop conjunctivitis and injection-site reactions compared with those who received placebo in the clinical trials. The MHRA noted that as dupilumab is a targeted therapy it is not associated with the same risks as general immunosuppression, but that the long term risks are unknown.
To access treatment via the early access scheme, eligible patients must have severe atopic dermatitis and have failed to respond to, be intolerant to or be ineligible for other therapies. Although the treatment is being investigated in children in clinical trials, the EAMS approval applies only to adults.
“Severe atopic eczema is a disease that has a major impact on quality of life and the treatment options available are limited and can have harmful effects,” states the MHRA in its report. “Under EAMS, dupilumab is being made available to those patients with the highest need who have run out of treatment options.”
Dupilumab was developed as part of an antibody collaboration between Regeneron and Sanofi that has been going since 2007. An application for marketing authorisation was filed with the European Medicines Agency in December 2016 and is currently under review.