Science Photo Library
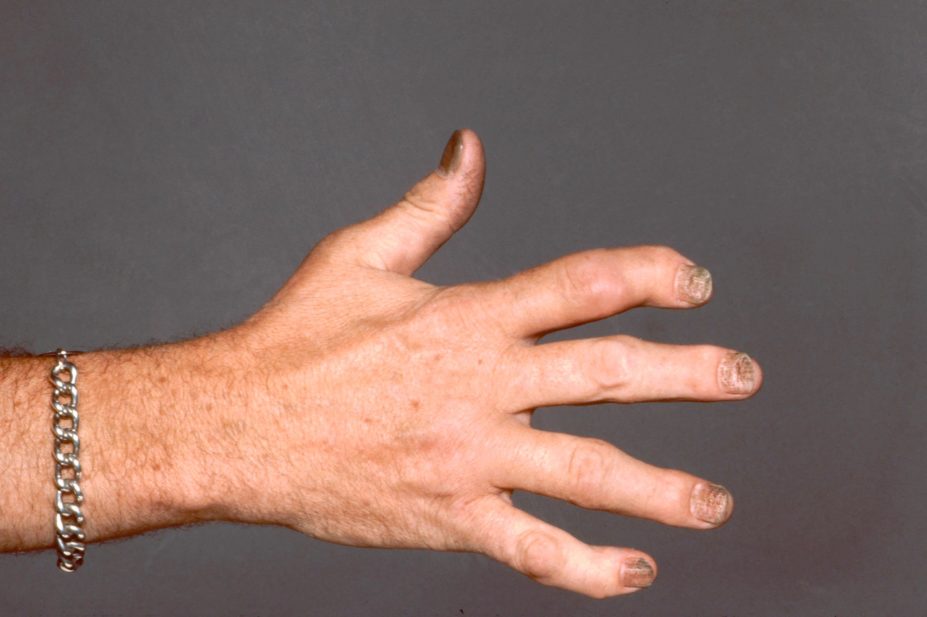
Biological therapies secukinumab and adalimumab are similarly effective at improving the musculoskeletal symptoms of psoriatic arthritis despite differing mechanisms of action, a head-to-head trial funded by Novartis and published in The Lancet suggests (9 May 2020)[1]
.
The multicentre, double-blind, active-controlled study enrolled 853 patients with psoriatic arthritis who were randomly assigned to receive 300mg of the IL-17A inhibitor, secukinumab, or 40mg of the anti-TNF agent, adalimumab, for 52 weeks. Treatment response was measured by the American College of Rheumatology (ACR) response criteria.
At week 52, the researchers found that a similar proportion of patients had at least a 20% improvement in the ACR response criteria in each group at 67% for secukinumab and 62% for adalimumab. The safety profiles were in accordance with previous data, although secukinumab was associated with a higher treatment retention rate.
Most patients with psoriatic arthritis are treated first-line with conventional disease-modifying therapy like methotrexate. But the researchers said there is a lack of data to inform the choice of biological therapy where methotrexate is ineffective or intolerable.
“This study presents a considerable volume of comparative efficacy and safety data on two biological agents with different mechanisms of action in the treatment of patients with psoriatic arthritis,” they concluded.
References
[1] McInnes I, Behrens F, Mease P et al. Lancet 2020;395:1496–1505. doi: 10.1016/S0140-6736(20)30564-X