John Bavosi / Science Photo Library
Proton pump inhibitors (PPIs) are among the most frequently prescribed drugs globally. Although they are cost effective when used appropriately, studies show they are prescribed without a clear indication in up to 70% of cases. Although the absolute risk of harm to individuals from PPIs is low, their widespread, long-term use can cause adverse effects that contribute to significant negative impacts at a population level. Action is required to limit inappropriate prescribing of PPIs and support deprescribing in patients on long-term therapy for whom the original indications no longer apply.
The rise of PPIs
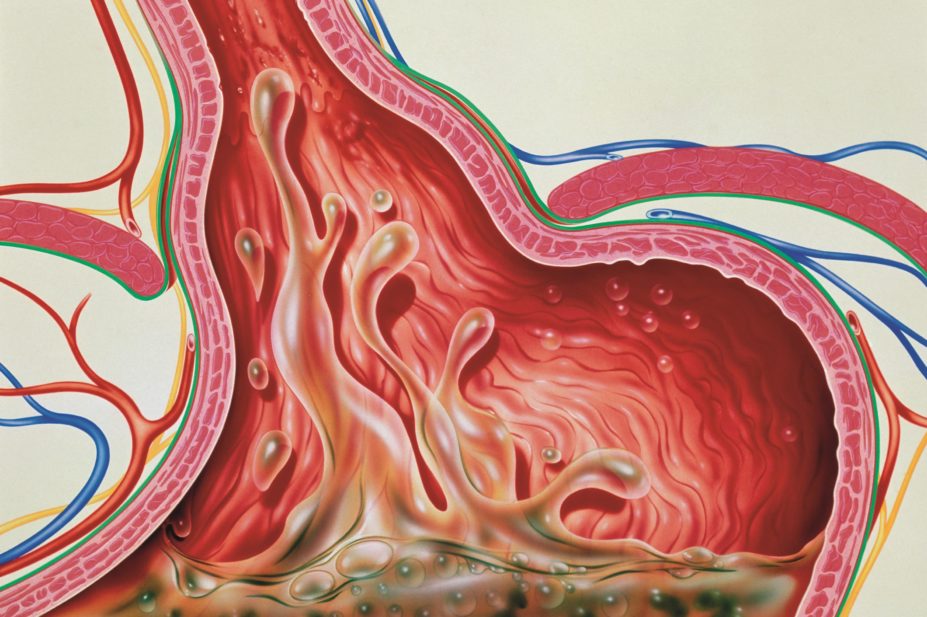
PPIs were introduced in the 1980s and rapidly became some of the bestselling medicines of all time. They inhibit gastric acid secretion through blockade of H+/K+-ATPases in parietal cells, and are highly effective for treating peptic ulceration, oesophagitis and gastro-oesophageal reflux[1],[2]
. They are also important components of Helicobacter pylori eradication regimens[3]
, and useful for prophylaxis against non-steroidal anti-inflammatory drug (NSAID) induced upper gastrointestinal injury[4]
. For most of these presentations, they are only intended for short-term use and are rarely required beyond four to eight weeks. In a minority of conditions (for example, severe Barrett’s oesophagus, gastrinoma and eosinophilic oesophagitis), protracted courses may be required[5]
.
PPIs combine high efficacy with low toxicity, and are perceived to be safe and cost effective[6]
. Consequently, they are widely prescribed. The total cost of PPIs to the UK NHS is more than £100m each year[6]
and global spend is in excess of £2bn. Five PPIs are currently licensed in the UK: omeprazole, lansoprazole, pantoprazole, esomeprazole and rabeprazole. In most cases, there is no clear evidence to support use of one over another, and class effects can be assumed[7]
.
Overprescribing is the norm
Studies consistently find that PPIs are overprescribed globally in both primary and secondary care. Their prevalence continues to increase: in Australia, prescriptions rose by 1,318% over one decade (1996–2006)[8]
. Most of this growth occurs in primary care, with increasing numbers of patients treated for longer durations[9]
. This is partly related to substitution of histamine-2 receptor antagonists, but the bulk represents expanded use of acid suppression therapy. A proportion of this increase is legitimate, on account of rising groups of patients treated with dual antiplatelet therapy for coronary artery or cerebrovascular disease, or bisphosphonates (for which PPIs partially mitigate risk of oesophagitis[10]
). There is also a small cohort of patients with chronic cough in whom gastro-oesophageal reflux is believed to be a contributor[11]
. The recent approvals of esomeprazole for general sale, and pantoprazole as an over-the-counter medicine, may further drive consumer use in the UK.
Some studies have examined the appropriateness of PPI prescriptions. In one study of older patients in Italy, 30% were taking a PPI with no clear indication, although a further 11% of this cohort possessed a recognised indication and were not on treatment (that is, PPIs were underprescribed)[12]
. A study of 124,133 first-time adult users from Denmark found that only one third met criteria for potential long-term use[13]
. In the same catchment population, only 4% of pre-existing long-term users (defined as more than 60 daily doses over a six-month period) had a diagnosis that merited long-term management[14]
.
Within hospitals, a retrospective study of surgical inpatients from the Netherlands identified non-compliance with guidelines in 46.6% of cases; 93.1% of these represented overprescribing[15]
. Audits of medical inpatients in the UK show inappropriate prescribing rates of 40.7–54.0%, of which 86.0% are cases of overprescribing[16]
,[17]
.
Other studies have probed the underlying rationale for PPI prescriptions and their continued use[18],[19],[20]
. The reasons are often questionable, and adherence to guidelines is poor despite educational and stewardship strategies[21]
. The most common explanations for long-term PPI use were inappropriate treatment of dyspepsia, prophylaxis for low-risk patients on NSAIDs or corticosteroids, and stress ulcer prophylaxis (in secondary care).
Communication is often poor. In one UK centre, suggested duration of treatment was specified in fewer than 20% of hospital discharge letters, less than one third indicated that prescriptions needed to be reviewed, and only half contained information explaining why the drug was started[22]
.
Overprescribing is more common in patients with comorbidities and polypharmacy who are likely to see several specialists, increasing the chance of a drug being prescribed and making it less likely that one clinician will take overall responsibility for the patient’s medicines.
Another problem is that once a patient has taken a PPI for longer than a few weeks, acid hypersecretion can occur on discontinuation. This causes rebound symptoms, and frequently establishes a vicious cycle of drug reinitiation and long-term continuation[23]
.
Possible harms
Over the past decade, many adverse effects of PPI therapy have been identified.
The most widely studied of these is Clostridium difficile infection. A meta analysis of 23 cohort and case-control studies, involving almost 300,000 patients, identified a 65% increase in the relative risk of C. difficile-associated diarrhoea[24]
. The mechanism remains unproven, but it may be that acid suppression permits viability of the C. difficile vegetative state, leading to clinically symptomatic infection. There are suggestions that PPIs also increase risks of Campylobacter and Salmonella gastroenteritis[25]
, and two studies demonstrate alterations in the gut microbiome[26]
.
Increased fracture risk has been reported with PPI use, most notably in the Nurses Health Study, which followed 79,899 female participants for eight years[27]
. The age-adjusted hazard ratio of hip fracture was 1.35 with more than two years of PPI use, and even higher in smokers. Possible explanations include reduced calcium absorption[28]
, or competition with osteoblast and osteoclast proton pumps that impedes bone remodelling[29]
. Deficiencies have also been reported in both iron[30]
and vitamin B12[31]
absorption, and a major issue in a small subset of patients is severe hypomagnesaemia[32]
. If the latter occurs, it is typically a drug class effect, likely caused by inhibition of cation transport in the colon.
PPIs are now well recognised as a cause of acute interstitial nephritis, which, in a nested case control study of 572,661 patients, occurred with an odds ratio of 5.16 (translating into an incidence of 11.98 per 100,000 person-years)[33]
. Early recognition and drug discontinuation are crucial for maximising renal recovery. There have also been reports of PPIs triggering subacute cutaneous lupus erythematosus[34]
.
Finally, increased rates of chronic kidney disease and myocardial infarction have been reported among PPI users. In one study of 173,321 patients, the hazard ratio of developing chronic renal impairment was 1.22, rising with increasing duration of exposure[35]
. In a further data-mining exercise examining records from 2.9 million patients, PPIs were associated with a 1.16-fold risk of myocardial infarction, and a 2-fold increased risk of cardiovascular mortality[36]
. The cause was not ascertained, and although the authors speculated about interference with nitric oxide signalling, an alternative explanation would be confounding because of increased use of acid suppression in patients with comorbidities and polypharmacy, and hence overall cardiovascular risk.
Drug interactions
Patients with multiple comorbidities and polypharmacy who take PPIs on a long-term basis are at high risk of drug-drug interactions. Specifically, an alteration of pH in the gastrointestinal tract can impact on drug absorption, and PPIs inhibit (to varying degrees) cytochrome (CYP) p450 and the p-glycoprotein pathway[37]
. This may be a particular issue for omeprazole, which has high affinity for CYP2C19 and moderate affinity for CYP3A4[38]
.
Drug interactions were raised as a major concern in the case of clopidogrel, which requires CYP2C19 for conversion to its active metabolite. An initial randomised controlled trial of 140 patients found a reduction in the platelet reactivity index after one week in patients receiving clopidogrel with omeprazole[39]
, and on this basis several regulatory agencies counselled that both omeprazole and esomeprazole should be avoided in this context. The clinical relevance of the interaction has, however, been called into question, with the prospective COGENT trial reporting no increase in cardiovascular events in 3,761 patients on omeprazole and dual antiplatelet therapy[40]
. The trial was terminated prematurely because of a lack of funding but a subsequent review concluded that, despite evidence of an ex vivo interaction, the case for adverse impact in patients in vivo had not been made[41]
.
Other research has highlighted a significant risk of bias in many retrospective studies that report harmful interactions between PPIs and clopidogrel[42]
, and showed that concomitant use halves the risk of gastrointestinal bleeding[43]
. Consequently, some authorities advocate preferential use of alternative PPIs with lower CYP2C19 affinity (such as pantoprazole or rabeprazole) in this patient cohort, although the effectiveness of this strategy has not been proven.
Although studies on drug interactions have been dominated by those focusing on antiplatelet therapies, PPIs can also decrease plasma concentrations of several antiretroviral agents, dabigatran, mycophenolate mofetil, and targeted oncological signal pathway inhibitors, as well as increase the concentrations of calcineurin inhibitors, methotrexate and metformin[44]
. As with clopidogrel, little evidence has emerged to date that these can cause clinically meaningful harm, although it would still be prudent to take care when prescribing PPIs with drugs that have potential for an interaction to occur.
Minimising overprescribing
The steps required to curb and subsequently reduce inappropriate prescribing include: recognition of the problem; use of alternative approaches to manage conditions currently treated “by default” with PPIs; education regarding appropriate indications and durations for their use; and enhanced drug stewardship akin to that employed widely for antimicrobials, mandating better documentation around PPI prescriptions and regular review. Patient involvement and shared decision-making are also essential[45]
.
One of the most frequent reasons for long-term PPI use is dyspepsia. This condition can often be ameliorated by medication rationalisation and lifestyle modification, such as weight loss or smoking cessation[2]
. Possible drug contributors to dyspepsia include calcium channel blockers, nitrates, theophyllines, bisphosphonates, antiplatelet agents and NSAIDs. A review should be conducted to consider whether these are still indicated or could be substituted. Where PPIs are prescribed, the lowest effective dose should be used for the shortest possible duration. The indications and intended durations should be clearly documented and communicated to the primary care provider and pharmacy.
Patients on long-term therapy should be reviewed at least annually. Substitution of PPIs with antacid or alginate therapy, or H2 receptor antagonists, can also be considered. Patients should be counselled on rebound acid secretion on drug discontinuation and on strategies to manage this (preferably without re-escalating PPI dose) and it should be explained that this does not necessarily represent recurrence of disease[23]
.
The other common rationale for long-term PPI prescribing is prophylaxis when receiving antiplatelet agents or NSAIDs. Most guidelines suggest this is only required for high-risk patients, specifically those aged over 65 years, with a history of peptic ulcer disease or upper gastrointestinal haemorrhage, or taking multiple medicines that augment gastrointestinal adverse effects[46],[47]
.
Time to deprescribe
Prescribing of PPIs has skyrocketed over the past decade. These drugs can be effective, but are principally intended for short-term use and yet are often not discontinued. There is clear and consistent evidence of overprescribing as clinicians overestimate benefits and underestimate harms, associated with substantial costs to healthcare providers. Measures should be put in place to educate prescribers on appropriate indications and durations for PPI use, provide a degree of stewardship, and facilitate long-term users in de-escalating therapy.
Daniel J B Marks is a clinical pharmacologist at University College London Hospital and a postdoctoral research fellow at the Centre for Molecular Medicine, University College London.
References
[1] Dworzynski K, Pollit V, Kelsey A et al. Management of acute upper gastrointestinal bleeding: summary of NICE guidance. The BMJ 2012;344:e3412. doi: 10.1136/bmj.e3412
[2] Ford AC & Moayyedi P. Dyspepsia. The BMJ 2013;347:f5059. doi: 10.1136/bmj.f5059
[3] Graham DY & Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010;59:1143–1153. doi: 10.1136/gut.2009.192757
[4] Laine L. GI risk and risk factors of NSAIDs. J Cardiovasc Pharmacol 2006;47:S60–66. PMID: 16785831
[5] Fitzgerald RC, di Pietro M, Ragunath K et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372
[6] Forgacs I & Loganayagam A. Overprescribing proton pump inhibitors. The BMJ 2008;336:2–3. doi: 10.1136/bmj.39406.449456.BE
[7] Neumann I, Letelier LM, Rada G et al. Comparison of different regimens of proton pump inhibitors for acute peptic ulcer bleeding. Cochrane Database Syst Rev 2013;(6):CD007999. doi: 10.1002/14651858.CD007999
[8] Hollingworth S, Duncan EL & Martin JH. Marked increase in proton pump inhibitors use in Australia. Pharmacoepidemiol Drug Saf 2010;19:1019–1024. doi: 10.1002/pds.1969
[9] Haastrup P, Paulsen MS, Zwisler JE et al. Rapidly increasing prescribing of proton pump inhibitors in primary care despite interventions: a nationwide observational study. Eur J Gen Pract 2014;20:290–293. doi: 10.3109/13814788.2014.905535
[10] McGowan B, Bennett K, Barry M et al. The utilisation and expenditure of medicines for the prophylaxis and treatment of osteoporosis. Ir Med J 2008;101:38–41. PMID: 18450246
[11] Ribolsi M, Savarino E, De Bortoli N et al. Reflux pattern and role of impedance-pH variables in predicting PPI response in patients with suspected GERD-related chronic cough. Aliment Pharmacol Ther 2014;40:966–973. doi: 10.1111/apt.12919
[12] Schepisi R, Fusco S, Sganga F et al. Inappropriate use of proton pump inhibitors in elderly patients discharged from acute care hospitals. J Nutr Health Aging 2016;20:665–670. doi: 10.1007/s12603-015-0642-5
[13] Haastrup PF, Rasmussen S, Hansen JM et al. General practice variation when initiating long-term prescribing of proton pump inhibitors: a nationwide cohort study. BMC Fam Pract 2016;17:57. doi: 10.1186/s12875-016-0460-9
[14] Haastrup PF, Paulsen MS, Christensen RD et al. Medical and non-medical predictors of initiating long-term use of proton pump inhibitors: a nationwide cohort study of first-time users during a 10-year period. Aliment Pharmacol Ther 2016;44:78–87. doi: 10.1111/apt.13649
[15] van den Bemt PM, Chaaouit N, van Lieshout EM et al. Noncompliance with guidelines on proton pump inhibitor prescription as gastroprotection in hospitalized surgical patients who are prescribed NSAIDs. Eur J Gastroenterol Hepatol 2016. doi: 10.1097/MEG.0000000000000634
[16] Batuwitage BT, Kingham JG, Morgan NE et al. Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad Med J 2007;83:66–68. doi: 10.1136/pgmj.2006.051151
[17] Jarchow-Macdonald AA & Mangoni AA. Prescribing patterns of proton pump inhibitors in older hospitalized patients in a Scottish health board. Geriatr Gerontol Int 2013;13:1002–1009. doi: 10.1111/ggi.12047
[18] Van Soest EM, Siersema PD, Dieleman JP et al. Persistence and adherence to proton pump inhibitors in daily clinical practice. Aliment Pharmacol Ther 2006;24:377–385. doi: 10.1111/j.1365-2036.2006.02982.x
[19] van Vliet EP, Otten HJ, Rudolphus A et al. Inappropriate prescription of proton pump inhibitors on two pulmonary medicine wards. Eur J Gastroenterol Hepatol 2008;20:608–612. doi: 10.1097/MEG.0b013e3282f52f95
[20] Heidelbaugh JJ, Goldberg KL & Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010;16:e228–234. PMID: 21250399
[21] van Vliet EP, Steyerberg EW, Otten HJ et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther 2009;29:213–221. doi: 10.1111/j.1365-2036.2008.03875.x
[22] Walker NM & McDonald J. An evaluation of the use of proton pump inhibitors. Pharm World Sci 2001;23:116–117. PMID: 11468876
[23] Reimer C, Sondergaard B, Hilsted L et al. Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology 2009;137:80–87. doi: 10.1053/j.gastro.2009.03.058
[24] Janarthanan S, Ditah I, Adler DG et al. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 2012;107:1001–1010. doi: 10.1038/ajg.2012.179
[25] Leonard J, Marshall JK & Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007;102:2047–2056. doi: 10.1111/j.1572-0241.2007.01275.x
[26] Imhann F, Bonder MJ, Vich Vila A et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376
[27] Khalili H, Huang ES, Jacobson BC et al. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. The BMJ 2012;344:e372. doi: 10.1136/bmj.e372
[28] Graziani G, Como G, Badalamenti S et al. Effect of gastric acid secretion on intestinal phosphate and calcium absorption in normal subjects. Nephrol Dial Transplant 1995;10:1376–1380. PMID: 8538929
[29] Costa-Rodrigues J, Reis S, Teixeira S et al. Dose-dependent inhibitory effects of proton pump inhibitors on human osteoclastic and osteoblastic cell activity. FEBS J 2013;280:5052–5064. doi: 10.1111/febs.12478
[30] McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol 2009;104:S5–9. doi: 10.1038/ajg.2009.45
[31] Lam JR, Schneider JL, Zhao W et al. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013;310:2435–2442. doi: 10.1001/jama.2013.280490
[32] Hess MW, Hoenderop JG, Bindels RJ et al. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther 2012;36:405–413. doi: 10.1111/j.1365-2036.2012.05201.x
[33] Blank ML, Parkin L, Paul C et al. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int 2014;86:837–844. doi: 10.1038/ki.2014.74
[34] Sandholdt LH, Laurinaviciene R & Bygum A. Proton pump inhibitor-induced subacute cutaneous lupus erythematosus. Br J Dermatol 2014;170:342–351. doi: 10.1111/bjd.12699
[35] Xie Y, Bowe B, Li T et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol 2016. doi: 10.1681/ASN.2015121377
[36] Shah NH, LePendu P, Bauer-Mehren A et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One 2015;10:e0124653. doi: 10.1371/journal.pone.0124653
[37] Polasek TM, Lin FP, Miners JO et al. Perpetrators of pharmacokinetic drug-drug interactions arising from altered cytochrome P450 activity: a criteria-based assessment. Br J Clin Pharmacol 2011;71:727–736. doi: 10.1111/j.1365-2125.2011.03903.x
[38] Li W, Zeng S, Yu LS et al. Pharmacokinetic drug interaction profile of omeprazole with adverse consequences and clinical risk management. Ther Clin Risk Manag 2013;9:259–271. doi: 10.2147/TCRM.S43151
[39] Gilard M, Arnaud B, Cornily JC et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008;51:256–260. doi: 10.1016/j.jacc.2007.06.064
[40] Bhatt DL, Cryer BL, Contant CF et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010;363:1909–1917. doi: 10.1056/NEJMoa1007964
[41] Focks JJ, Brouwer MA, van Oijen MG et al. Concomitant use of clopidogrel and proton pump inhibitors: impact on platelet function and clinical outcome- a systematic review. Heart 2013;99:520–527. doi: 10.1136/heartjnl-2012-302371
[42] Lima JP & Brophy JM. The potential interaction between clopidogrel and proton pump inhibitors: a systematic review. BMC Med 2010;8:81. doi: 10.1186/1741-7015-8-81
[43] Siller-Matula JM, Jilma B, Schror K et al. Effect of proton pump inhibitors on clinical outcome in patients treated with clopidogrel: a systematic review and meta-analysis. J Thromb Haemost 2010;8:2624–2641. doi: 10.1111/j.1538-7836.2010.04049.x
[44] Yucel E, Sancar M, Yucel A et al. Adverse drug reactions due to drug-drug interactions with proton pump inhibitors: assessment of systematic reviews with AMSTAR method. Expert Opin Drug Saf 2016;15:223–236. doi: 10.1517/14740338.2016.1128413
[45] Reeve E, Shakib S, Hendrix I et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmacol 2014;78:738–747. doi: 10.1111/bcp.12386
[46] Bhatt DL, Scheiman J, Abraham NS et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol 2008;103:2890–2907. doi: 10.1111/j.1572-0241.2008.02216.x
[47] Lanza FL, Chan FK, Quigley EM et al. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol 2009;104:728–738. doi: 10.1038/ajg.2009.115